Difference between revisions of "Drug-induced liver disease"
Jump to navigation
Jump to search
(split out) |
|||
| Line 73: | Line 73: | ||
*[http://livertox.nih.gov Drug-liver toxicity (nih.gov)]. | *[http://livertox.nih.gov Drug-liver toxicity (nih.gov)]. | ||
[[Category:Medical liver | [[Category:Medical liver disease]] | ||
Revision as of 15:05, 5 September 2014
Drug-induced liver disease, also drug-induced liver toxicity, is relatively common.
Drug reactions in general are dealt with in drug toxicity.
General
- Drugs can do almost anything; may include: granulomata, bile duct loss, cholestasis, ischemic type injury.
- Effects can be delayed -- temporal relationship not always obvious.
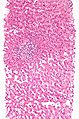
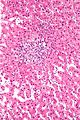
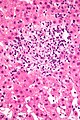
Microscopic
Features:
- Non-specific findings.
- +/-Eosinophils[1] - significant suggestive finding.
- +/-Steatosis - periportal macrovesicular, microvesicular.
- +/-Vanishing bile duct syndrome.
- +/-Granulomas.
Images
Specific patterns
Acute hepatits:
- Related to Rx - most often antibiotics.
- Amiodarone - cardiac arrhythmias.
- Tamoxifen - breast cancer.
- Carbamazepine - seizures.
- Venlafaxine - depression.[3]
- Thalidomide - multiple myeloma.[4]
Specific drugs
Acetaminophen:
- Zone 3 necrosis.
Methotrexate - chronic use:
- Histology:[7]
- Features of steatohepatitis.
- Zone III steatosis.
- Ballooning degeneration.
- Portal inflammation with mixed population (lymphocytes, macrophages, PMNs).
- Nuclear atypia (hyperchromasia, pleomorphism, vacuolation).
- Described as just nuclear size variation by some.[8]
- Features of steatohepatitis.
Sign out
LIVER, MEDICAL CORE BIOPSIES: - MILD STEATOHEPATITIS AND MILD FIBROSIS (1/4). - MILD TO MODERATE STEATOSIS. COMMENT: The findings are compatible with nonalcoholic steatohepatitis (NASH), alcoholic steatohepatitis (ASH) or drug effect. The steatosis is periportal predominant; this is not typical for NASH or ASH. Clinical correlation and review of medications is suggested.
See also
References
- ↑ Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 166. ISBN 978-0470519035.
- ↑ Grieco, A.; Forgione, A.; Miele, L.; Vero, V.; Greco, AV.; Gasbarrini, A.; Gasbarrini, G.. "Fatty liver and drugs.". Eur Rev Med Pharmacol Sci 9 (5): 261-3. PMID 16237810.
- ↑ Stadlmann, S.; Portmann, S.; Tschopp, S.; Terracciano, LM. (Nov 2012). "Venlafaxine-induced cholestatic hepatitis: case report and review of literature.". Am J Surg Pathol 36 (11): 1724-8. doi:10.1097/PAS.0b013e31826af296. PMID 23073329.
- ↑ Vilas-Boas, F.; Gonçalves, R.; Sobrinho Simões, M.; Lopes, J.; Macedo, G. (Oct 2012). "Thalidomide-induced acute cholestatic hepatitis: case report and review of the literature.". Gastroenterol Hepatol 35 (8): 560-6. doi:10.1016/j.gastrohep.2012.05.007. PMID 22789729.
- ↑ 5.0 5.1 Millea PJ (August 2009). "N-acetylcysteine: multiple clinical applications". Am Fam Physician 80 (3): 265–9. PMID 19621836.
- ↑ URL: http://www.mskcc.org/mskcc/html/69310.cfm. Accessed on: 19 October 2010.
- ↑ Burt, Alastair D.;Portmann, Bernard C.;Ferrell, Linda D. (2006). MacSween's Pathology of the Liver (5th ed.). Churchill Livingstone. pp. 715. ISBN 978-0-443-10012-3.
- ↑ MG. 23 September 2009.