Difference between revisions of "Endometrial hyperplasia"
(→Images) |
|||
| Line 164: | Line 164: | ||
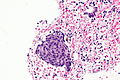
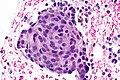
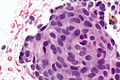
Image: Squamous morule 2 - endometrium -- very high mag.jpg | SM - very high mag. | Image: Squamous morule 2 - endometrium -- very high mag.jpg | SM - very high mag. | ||
Image: Squamous morule 2 - endometrium -- extremely high mag.jpg | SM - extremely high mag. | Image: Squamous morule 2 - endometrium -- extremely high mag.jpg | SM - extremely high mag. | ||
</gallery> | |||
<gallery> | |||
Image: Endometrial polyp with fused glands -- low mag.jpg | Endometrial polyp with fused glands - low mag. (WC) | |||
Image: Endometrial polyp with fused glands -- intermed mag.jpg | Endometrial polyp with fused glands - intermed. mag. (WC) | |||
Image: Endometrial polyp with fused glands -- high mag.jpg | Endometrial polyp with fused glands - high mag. (WC) | |||
</gallery> | </gallery> | ||
Revision as of 04:08, 12 December 2013
- See Endometrium for an introduction to the topic.
Endometrial hyperplasia, abbreviated EH, is a precursor to endometrial carcinoma.
Overview
The most widely used system is from the World Health Organization (WHO).
WHO classification - overview
The WHO system is based on determining:
- Gland density (normal = simple hyperplasia, high density = complex hyperplasia).
- Presence/absence of nuclear atypia.
Alternate classifications - overview
Two alternative grading systems exist, that are (currently) not widely used:[1]
- European group of experts (1999).
- Endometrial collaborative group/Harvard (2000).
Both consist of two categories, as opposed to four found in the WHO classification.
European group of experts classification
- Endometrial hyperplasia.
- Endometrioid neoplasia.
Endometrial collaborative group/Harvard classification
- Endometrial hyperplasia.
- Endometrial intraepithelial neoplasia (EIN).
WHO classification
Management of endometrial hyperplasia
- Endometrial hyperplasia with atypia is usually treated with hysterectomy.[2]
- In women who want to maintain fertility it may be treated with progestin + short interval re-biopsies (q3 months).[3]
- Endometrial hyperplasia without atypia is treated by:
- Progestins + close follow-up OR hysterectomy.
Risk of progression to carcinoma
Approximate risk of progression to endometrial carcinoma - Latta rule of 3s:[4]
| Simple | Complex | |
| Without atypia | 1% | 3% |
| With atypia | 9% † | 27% ‡ |
Notes:
Ki-67
There is one paper that looks at Ki-67:[6]
| Diagnosis | Percent positive |
|---|---|
| Secretory phase endometrium | |
| Proliferative phase endometrium | |
| Simple hyperplasia | |
| Simple hyperplasia with atypia | |
| Complex hyperplasia | |
| Complex hyperplasia with atypia |
WHO system
Almost all hyperplasia is seen in the context of proliferative-type endometrium. Hyperplasia in the secretory-type endometrium is extremely rare and something diagnosed by or in consultation with an expert in gynecologic pathology.
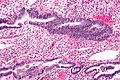
Simple endometrial hyperplasia
- AKA simple hyperplasia.
General
- More common than simple endometrial hyperplasia with atypia.
- Very low risk for progressing to endometrioid endometrial carcinoma.
Microscopic
Features:[7]
- Irregular dilated glands (with large lumens) - key feature.
- Glands described as "animal shapes".
- Variation of gland size.
- No nuclear atypia.
- Uniform columnar nuclei.
- Normal gland density (gland area in plane of section/total area ~= 1/3).
DDx:
- Disordered proliferative phase.
- Complex endometrial hyperplasia - has increased gland-to-stroma ratio.
- Cystic atrophy of the endometrium - does not have proliferative activity.[8]
- Benign endometrial polyp - has thick-walled blood vessels; simple endometrial hyperplasia should not be diagnosed in a polyp.[8]
Images:
Simple endometrial hyperplasia with atypia
General
- Very uncommon.
Microscopic
Features:[7]
- Irregular dilated glands (with large lumens) - important feature.
- Glands described as "animal shapes".
- Variation of gland size.
- No nuclear atypia.
- Uniform columnar nuclei.
- Normal gland density (gland area in plane of section/total area ~= 1/3).
- Nuclear atypia:[9]
- Loss of basal nuclear stratification.
- Nuclear size variation.
- Nuclear rounding.
- Nuclei lacking atypical = uniform columnar nuclei.
- Nucleoli.
- Hyperchromasia or vesicular nuclei.
Notes:
- There are no clear criteria for atypia. Different sources list different features.
- VL criteria for atypia (all should be present):
- Increased NC ratio.
- Atypical: ~ 1:2
- Not atypical: ~1:3.
- Oval nuclei with small major axis to minor axis ratio.
- Atypical: major axis:minor axis = <=2:1.
- Not atypical: major axis:minor axis = >=3:1
- NB: round nuclei: major axis:minor axis = 1:1.
- Small nucleoli (~1/5 the size of the nucleus).
- Increased NC ratio.
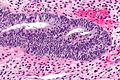
Complex endometrial hyperplasia
| Complex endometrial hyperplasia | |
|---|---|
| External resources | |
| EHVSC | 10169 |
Microscopic
Features:
- Increase in size & number of glands + irregular shape - key feature.
- Cell stratification.
- Nuclear enlargement.
- Mitoses common.
- No nuclear atypia.
Notes:
- Normal "gland-to-stroma ratio" is 1:3.
- Two "touching" glands may be one gland in section.
DDx:
- Complex endometrial hyperplasia with atypia.
- Endometrioid endometrial carcinoma - see endometrial carcinoma versus complex endometrial hyperplasia.
Images
Squamous morules - commonly associated with hyperplasia and malignancy:
Endometrial carcinoma versus complex endometrial hyperplasia
Complex endometrial hyperplasia:
- Non-confluent - glands distinct from one another.
Classic criteria for endometrial carcinoma
This is pimping material that shows up on exams.
Endometrial carcinoma has one of the following:[10][11][12]
- Desmoplastic stromal response.
- Confluent cribriform growth. †
- Extensive papillary growth. †
- Severe cytologic atypia. †
Note:
- † There is a size cut-off for criteria 2, 3 and 4: > 2.1 mm.[11]
How to remember ABCDE:
- Atypia Bad.
- Confluent cribriform growth.
- Desmoplasia.
- Extensive papillary growth.
Sign out
ENDOMETRIUM, BIOPSY: - COMPLEX ENDOMETRIAL HYPERPLASIA. -- NEGATIVE FOR CYTOLOGIC ATYPIA.
ENDOMETRIUM, BIOPSY: - SMALL FOCUS OF COMPLEX ENDOMETRIAL HYPERPLASIA WITHOUT ATYPIA, WITH SQUAMOUS MORULES. - ENDOMETRIAL POLYP WITH ONE ATYPICAL GLAND AND A SQUAMOUS MORULE. - SCANT ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY.
Complex endometrial hyperplasia with atypia
| Complex endometrial hyperplasia with atypia | |
|---|---|
| External resources | |
| EHVSC | 10181 |
- AKA complex atypical hyperplasia.
General
- High risk of transformation to endometrial carcinoma.
Microscopic
Features:
- Increase in size & number of glands + irregular shape - key feature.
- Cell stratification.
- Nuclear enlargement.
- Nuclear atypia:
- Round nuclei ~ 2-3x the size of a lymphocyte.
- Grey/translucent chromatin.
- Nucleoli.
- Mitoses common.
Note:
- Atypical nuclei often hide between non-typical nuclei, like peg cells in the fallopian tube.
DDx:
- Complex endometrial hyperplasia.
- Endometrioid endometrial carcinoma - see endometrial carcinoma versus complex endometrial hyperplasia.
Image:
Sign out
Insufficient confluence for carcinoma
ENDOMETRIUM, BIOPSY: - COMPLEX ENDOMETRIAL HYPERPLASIA WITH ATYPIA, SEE COMMENT. COMMENT: The sections show architecturally complex crowded glands with focal morular squamous metaplasia and focal cribriforming. Desmoplasia is not identified. The degree of gland confluence is not considered sufficient for the diagnosis of endometrial carcinoma. Nuclear atypia is present focally.
Insufficient extent for carcinoma
ENDOMETRIUM, BIOPSY: - COMPLEX ENDOMETRIAL HYPERPLASIA WITH ATYPIA, SEE COMMENT. COMMENT: The sections show architecturally complex back-to-back glands with focal morular squamous metaplasia and cribriforming. Desmoplasia is not present. The extent, i.e. the size of the abnormality, is not considered sufficient for the diagnosis of endometrial carcinoma.
Other
Endometrial hyperplasia with secretory changes
General
- Rare.
- Secretory changes seen in 1-2% of endometrial hyperplasias/endometrial carcinomas.[13]
Microscopic
Features:[14]
- Secretory changes - includes at least one of three following:[15]
- Stromal decidualization.
- Cytoplasmic vacuolization.
- Intraluminal secretions.
- Proliferative-type epithelium. †
- Mitoses.
- Nuclear atypia.
- Pseudostratified epithelium.
Notes:
- † This is not precisely defined. I suppose it is some of the things Bell and Ostrezega[16] mention (mitoses, nuclear atypia, pseudostratified epithelium).
- Bell and Ostrezega[16] give a laundry list for differentiating benign secretory endometrium from hyperplasia with secretory changes: focal architectural abnormalities, metaplastic ciliated & "clear" cells, sharp luminal border, epithelial pseudopalisading, nuclear atypia, vesicular nuclei, mitoses.
DDx:
Images:
See also
References
- ↑ Dietel, M. (Nov 2001). "The histological diagnosis of endometrial hyperplasia. Is there a need to simplify?". Virchows Arch 439 (5): 604-8. PMID 11764378.
- ↑ URL: http://www.aafp.org/afp/990600ap/3069.html.
- ↑ URL: http://www.aafp.org/afp/20060801/practice.html.
- ↑ Latta, E. January 2009.
- ↑ Jump up to: 5.0 5.1 Kurman, RJ.; Kaminski, PF.; Norris, HJ. (Jul 1985). "The behavior of endometrial hyperplasia. A long-term study of untreated hyperplasia in 170 patients.". Cancer 56 (2): 403-12. PMID 4005805.
- ↑ Abike, F.; Tapisiz, OL.; Zergeroglu, S.; Dunder, I.; Temizkan, O.; Temizkan, I.; Payasli, A. (2011). "PCNA and Ki-67 in endometrial hyperplasias and evaluation of the potential of malignancy.". Eur J Gynaecol Oncol 32 (1): 77-80. PMID 21446331.
- ↑ Jump up to: 7.0 7.1 Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 236. ISBN 978-0443069208.
- ↑ Jump up to: 8.0 8.1 McCluggage, WG. (Aug 2006). "My approach to the interpretation of endometrial biopsies and curettings.". J Clin Pathol 59 (8): 801-12. doi:10.1136/jcp.2005.029702. PMC 1860448. PMID 16873562. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860448/.
- ↑ Silverberg, SG. (Mar 2000). "Problems in the differential diagnosis of endometrial hyperplasia and carcinoma.". Mod Pathol 13 (3): 309-27. doi:10.1038/modpathol.3880053. PMID 10757341.
- ↑ Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 239. ISBN 978-0443069208.
- ↑ Jump up to: 11.0 11.1 Kurman, RJ.; Norris, HJ. (Jun 1982). "Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well-differentiated carcinoma.". Cancer 49 (12): 2547-59. PMID 7074572.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/Endometrium_11protocol.pdf. Accessed on: 12 January 2012.
- ↑ Simon RA, Hansen K, Xiong JJ, et al. PTEN status and frequency of endometrial carcinoma and its precursors arising in functional secretory endometrium; an immunohistochemical study of 29 cases. Mod Pathol. 2012;25(Suppl 2): 1248A.
- ↑ Simon RA. CAP Today. June 2012. Accessed on: 24 April 2013.
- ↑ Tresserra, F.; Lopez-Yarto, M.; Grases, PJ.; Ubeda, A.; Pascual, MA.; Labastida, R. (Mar 2003). "Endometrial hyperplasia with secretory changes.". Gynecol Oncol 88 (3): 386-93. PMID 12648591.
- ↑ Jump up to: 16.0 16.1 Bell, CD.; Ostrezega, E. (Aug 1987). "The significance of secretory features and coincident hyperplastic changes in endometrial biopsy specimens.". Hum Pathol 18 (8): 830-8. PMID 3610133.