Ablated endometrium
(Redirected from Endometrial ablation)
Jump to navigation
Jump to search
| Ablated endometrium | |
|---|---|
| Diagnosis in short | |
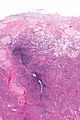
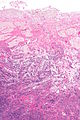
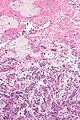
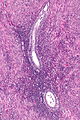
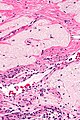
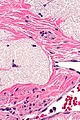
 Ablated_endometrium. H&E stain. | |
|
| |
| LM | endometrium completely or partially replaced by hyaline material and fibrosis |
| LM DDx | implantation site |
| Site | endometrium |
|
| |
| Associated Dx | endometriosis (in hysterectomy specimens) |
| Clinical history | history of ablation, dysfunctional uterine bleeding |
| Symptoms | none |
| Prevalence | common |
| Prognosis | benign |
| Treatment | none |
Ablated endometrium, also endometrium post-ablation procedure, is endometrium that has been destroyed to render it nonfunctional.
Endometrial ablation is done to treat menorrhagia and metromenorrhagia in women that are done with their childbearing.
General
- Alternative to hysterectomy.
- Thermal injury - delivered via balloon.
- High prevalence of endometriosis in individuals with hysterectomy post-ablation procedure.[1]
Notes:
- NovaSure is a commonly used ablation system.[2]
Microscopic
Features:
- Endometrium completely or partially replaced by:
- Hyaline material, fibrosis.
DDx:
Images
Sign out
UTERUS, UTERINE CERVIX, TOTAL HYSTERECTOMY: - SUPERFICIAL FIBROSIS AND HYALINE CHANGE OF THE UTERINE LINING -- COMPATIBLE WITH PRIOR ABLATION. - UTERUS WITH LEIOMYOMAS. - UTERINE CERVIX WITHIN NORMAL LIMITS.
See also
References
- ↑ Riley, KA.; Davies, MF.; Harkins, GJ.. "Characteristics of patients undergoing hysterectomy for failed endometrial ablation.". JSLS 17 (4): 503-7. doi:10.4293/108680813X13693422520602. PMID 24398189.
- ↑ Gimpelson, RJ. (2014). "Ten-year literature review of global endometrial ablation with the NovaSure® device.". Int J Womens Health 6: 269-280. doi:10.2147/IJWH.S56364. PMID 24648771.