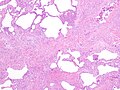
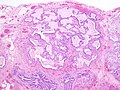
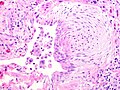
Usual interstitial pneumonia
Jump to navigation
Jump to search
| Usual interstitial pneumonia | |
|---|---|
| Diagnosis in short | |
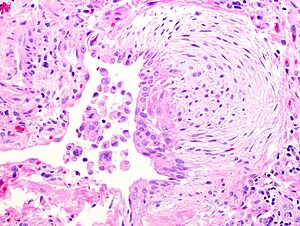 Fibroblast focus in usual interstitial pneumonia. H&E stain. | |
| LM DDx | asbestosis, chronic hypersensitivity pneumonitis, collagen vascular disease (e.g. systemic lupus erythematosus, rheumatoid arthritis, scleroderma), chronic drug toxicity |
| Stains | iron stain -ve |
| Gross | lower lobe predominant fibrosis |
| Site | lung - see diffuse lung diseases |
|
| |
| Signs | signs of right heart failure |
| Symptoms | shortness of breath |
| Prevalence | uncommon |
| Radiology | interstitial pattern, lower lobe predominant |
| Prognosis | usually poor, dependent on amount of fibrosis |
| Other | histologic correlate of idiopathic pulmonary fibrosis |
| Clin. DDx | asbestosis, chronic hypersensitivity pneumonitis, collagen vascular disease (history missing), chronic drug toxicity (history missing) |
| Treatment | lung transplantation |
Usual interstitial pneumonia, abbreviated UIP, is common diffuse lung disease. Overall, it is uncommon.
General
- It is sometimes used incorrectly as a synonym for idiopathic pulmonary fibrosis. It is a histomorphologic pattern and has a DDx (see below).
- UIP cannot be diagnosed via bronchoscopic or transbronchial biopsy,[1] as it is peripheral.
Epidemiology
- Disease of the old - rare in under 50 years old.[2]
- Dismal prognosis - mean survival after diagnosis ~ 2.8 years.[3]
Radiology
- Honeycombing - multiple defects that obliterate the normal lung architecture - multiple spherical voids in the lung parenchyma; radiologically these are seen as lucencies.[4]
- Usually subplural, i.e. peripheral lung.
- Classically lower lobe predominant.
- Traction bronchiectasis.
Note:
- Cysts - have thin walls (think of emphysema, lymphangioleiomyomatosis et cetera).
- Cysts may be isolated/not close to a neighbour.
- Medcyclopaedia defines it as: thin-walled, well-demarcated and >1 cm.[5]
Microscopic
Features:[6]
- Fibroblast foci:
- Interstitial inflammation.
- Microscopic honeycombing.
- Typically peripheral - cysts lined by ciliated epithelium.
- Spatial heterogeneity - patchy lesional distribution (areas of abnormal and normal lung may appear beside one another).
- Temporal heterogeneity - lesions of differing age side-by-side.[9]
Notes:
- Disease worse distant from large airways: lower lung field predominance, typically worse at periphery of lobule and lung.[10]
- Heterogeneity of inflammation: airspace macrophages & inflammation minimal in honeycombed foci.
DDx of UIP:[11]
- Idiopathic pulmonary fibrosis (UIP not otherwise specified).
- Asbestosis = UIP pattern + ferruginous bodies with asbestos fibers.
- Chronic hypersensitivity pneumonitis (AKA extrinsic allergic alveolitis) - classically centrilobular predominant +/- granulomas.
- Collagen vascular disease - includes systemic lupus erythematosus, rheumatoid arthritis, scleroderma.[12]
- Chronic drug toxicity.[13]
Images
See also
References
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 186. ISBN 978-0443066313.
- ↑ AC UBC S.102.
- ↑ Bjoraker, JA.; Ryu, JH.; Edwin, MK.; Myers, JL.; Tazelaar, HD.; Schroeder, DR.; Offord, KP. (Jan 1998). "Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis.". Am J Respir Crit Care Med 157 (1): 199-203. PMID 9445300.
- ↑ http://www.medcyclopaedia.com/library/topics/volume_v_1/h/honeycombing.aspx
- ↑ http://www.medcyclopaedia.com/library/topics/volume_v_1/l/lung_cyst.aspx
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 186-9. ISBN 978-0443066313.
- ↑ http://www.epler.com/IPFWhat%27sIPFDiseaseInformation2.htm
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 189. ISBN 978-0443066313.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 92. ISBN 978-0781765275.
- ↑ A. Churg. UBC S.103.
- ↑ Wick, Mark R.; Leslie, Kevin (2005). Practical pulmonary pathology: a diagnostic approach. Edinburgh: Churchill Livingstone. ISBN 0-443-06631-0. OCLC 156861539.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 374. ISBN 978-1416054542.
- ↑ Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC (2000). "Pulmonary drug toxicity: radiologic and pathologic manifestations". Radiographics : a review publication of the Radiological Society of North America, Inc 20 (5): 1245-59. PMID 10992015.