Proliferative phase endometrium
Jump to navigation
Jump to search
| Proliferative phase endometrium | |
|---|---|
| Diagnosis in short | |
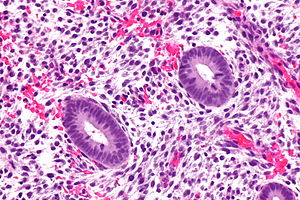 Proliferative endometrium. H&E stain. | |
|
| |
| LM | round spaced pseudostratified glands, mitoses (stroma and glands), normal gland-to-stroma ratio, no glandular dilation |
| LM DDx | disordered proliferative phase, simple endometrial hyperplasia, complex endometrial hyperplasia, early secretory phase endometrium |
| Site | endometrium |
|
| |
| Prevalence | common - normal finding |
Proliferative phase endometrium, abbreviated PPE, is a very common diagnosis in endometrial specimens.
General
- Day 1-13 in the protypical menstrual cycle of 28 days.
- May be day 5-13 - if the menstruation is not included.
- "Exodus" pattern is a term used to describe exfoliation of endometrial cells during the proliferative phase.
- On pap tests this is associated with the classic double contoured balls of endometrial epithelium and stroma.
Note:
- Proliferative phase = follicular phase.
- Gynecologists prefer the ovarian descriptor, i.e. follicular phase; pathologists go by what they see, i.e. proliferative endometrium.
- When the patient is >40 years, some advocate the use of the term proliferative type endometrium (instead of the term proliferative endometrium).[1]
Gross
- Thickened endometrium.
Microscopic
Features:[2]
- Glands:
- Straight, tubular, composed of tall pseudostratified columnar cells - key feature.
- Mitotic figures - key feature. †
- Stroma:
- Cellular stroma (spindle cells).
- Mitoses.
- Usually harder to find than in the glands.
Notes:
- † McCluggage says one shouldn't call PPE without mitoses, as some pseudostratification can be seen in atrophic endometrium.[2]
- There is no guidance on how hard one should look. VL suggests searching ~ 10 mm^2 with the 20x objective. This represents approximately ~ 10 fields of view with a microscope that has a 22 mm eye piece.
- Significant negatives:
- No vacuolation.
- No mucus secretion.
- Inflammation (neutrophils, rare plasma cell) & stromal breakdown common early in the proliferative phase.[3]
DDx:
- Endometrial polyp.
- Disordered proliferative endometrium.
- Endometrial hyperplasia:
- Secretory phase endometrium, early - >=50% of gland have subnuclear vacuoles and >=50% of cells in the glands have subnuclear vacuoles.[4]
Images:
Sign out
ENDOMETRIUM, BIOPSY: - PROLIFERATIVE PHASE ENDOMETRIUM.
ENDOMETRIUM, ASPIRATION: - PROLIFERATIVE PHASE ENDOMETRIUM.
ENDOMETRIUM, BIOPSY: - PROLIFERATIVE PHASE ENDOMETRIUM. - ENDOCERVICAL MUCOSA AND STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS.
ENDOMETRIUM, BIOPSY: - PROLIFERATIVE ENDOMETRIUM, FOCALLY WITH A FIBROTIC STROMA. - BENIGN STRIPPED ENDOCERVICAL EPITHELIUM. - NEGATIVE FOR HYPERPLASIA AND NEGATIVE FOR MALIGNANCY.
ENDOMETRIUM, ASPIRATION: - EARLY PROLIFERATIVE PHASE ENDOMETRIUM WITH SOME SHEDDING (APOPTOTIC CELLS, INFILTRATING NEUTROPHILS, BALLS OF CONDENSED ENDOMETRIAL STROMA). - SCANT STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - NEGATIVE FOR HYPERPLASIA.
Not quite normal
ENDOMETRIUM, BIOPSY: - EARLY SECRETORY PHASE ENDOMETRIUM. - FOCUS OF CROWDED PROLIFERATIVE GLANDS, SEE COMMENT. COMMENT: There is a small focus of crowded and irregular proliferative glands without cytologic atypia. The possibility of a polyp is considered but the vessels and polyp-type stroma are lacking. Suggest clincal follow up with a consideration of a repeat biopsy in 3 to 6 months to rule out a hyperplastic lesion.
Post-menopausal
ENDOMETRIUM, BIOPSY: - PROLIFERATIVE TYPE ENDOMETRIUM. -- NEGATIVE FOR HYPERPLASIA. -- NEGATIVE FOR MALIGNANCY.
ENDOMETRIUM, BIOPSY: - PROLIFERATIVE-TYPE ENDOMETRIUM, LIMITED STROMA PRESENT. - STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - NO EVIDENCE OF HYPERPLASIA.
Micro
The sections show endometrium with proliferative glands without significant dilation or irregularity of shape. The gland-to-stroma ratio is within normal limits. Mitotic activity is mild. No nuclear atypia is apparent.
See also
References
- ↑ Gardiner G. January 2009.
- ↑ 2.0 2.1 McCluggage, WG. (Aug 2006). "My approach to the interpretation of endometrial biopsies and curettings.". J Clin Pathol 59 (8): 801-12. doi:10.1136/jcp.2005.029702. PMC 1860448. PMID 16873562. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860448/.
- ↑ Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 197. ISBN 978-0443069208.
- ↑ Mazur, Michael T.; Kurman, Robert J. (2005). Diagnosis of Endometrial Biopsies and Curettings: A Practical Approach (2nd ed.). Springer. pp. 14. ISBN 978-0387986159.
- ↑ URL: http://www.cytochemistry.net/microanatomy/medical_lectures/oviduct_and_uterus.htm. Accessed on: 23 October 2012.