Urothelial carcinoma in situ
Jump to navigation
Jump to search
Urothelial carcinoma in situ, also known as high-grade (urothelial) dysplasia, a non-invasive urothelial neoplasm without papillae.
| Urothelial carcinoma in situ | |
|---|---|
| Diagnosis in short | |
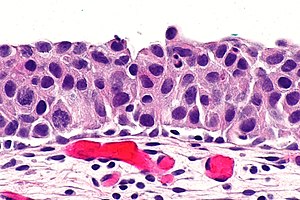 Urothelial carcinoma in situ. H&E stain. | |
|
| |
| Synonyms | urothelial cell carcinoma in situ, high-grade dysplasia |
|
| |
| LM | nuclear changes (enlargement of nuclei (often 4-5x the size of stromal lymphocytes), nuclear pleomorphism - marked variation in size of nuclei), +/-disordered arrangement/crowding of cells, +/-mitoses, +/-enlarged nucleoli |
| LM DDx | urothelial carcinoma, urothelial dysplasia, urothelial atypia of unknown significance |
| IHC | CK20 +ve (full thickness), Ki-67 high, p53 +ve, CD44 -ve, CK7 +ve |
| Site | urothelium - urinary bladder, ureter, renal pelvis, prostatic urethra |
|
| |
| Signs | +/-hematuria |
| Prevalence | relatively uncommon |
| Endoscopy | erythema or edema, may be unremarkable |
| Clin. DDx | invasive (flat) urothelial carcinoma, inflammation (cystitis) |
It is also known as carcinoma in situ (abbreviated CIS) and urothelial cell carcinoma in situ (abbreviated UCC in situ). Urothelial carcinoma in situ may be abbreviated UCIS.
General
- Lack papillae.
- Uncommon in relation to other urothelial lesions.
- Less common than invasive flat urothelial carcinoma ~3-4x more common than UCIS.[1]
Classification of flat urothelial lesions
The World Health Organization classification is:[2]
- Reactive urothelial atypia.
- Flat urothelial hyperplasia.
- Urothelial atypia of unknown significance.
- Urothelial dysplasia (low-grade dysplasia).
- Urothelial carcinoma in situ (high-grade dysplasia).
- Invasive urothelial carcinoma.
Gross
- Flat lesion - erythema or edema, may be unremarkable.[3]
Microscopic
Features:
- Nuclear changes key feature.
- Enlargement of nuclei (often 4-5x the size of stromal lymphocytes) -- diagnostic.[4]
- Normal urothelium approx. 2x the size of stromal lymphocytes.
- Nuclear pleomorphism - marked variation in size of nuclei.
- Enlargement of nuclei (often 4-5x the size of stromal lymphocytes) -- diagnostic.[4]
- +/-Disordered arrangement/crowding of cells.
- In normal urothelium the cell line-up on the basement membrane.
- Umbrella cells often absent.
- +/-Mitoses present.
- +/-Enlarged nucleoli.
Note:
- The urothelium may be "depleted", i.e. exist only of rare large cells on the basement membrane.
- This is known as clinging urothelial carcinoma in situ.[5]
DDx:
- Urothelial atypia of unknown significance - waffle diagnosis.
- Urothelial dysplasia.
- Urothelial carcinoma, invasive.
Images
www:
IHC
- CK20 +ve in deep cells.
- Normal urothelium -- only the umbrella cells.
- p53 +ve.
- CD44 -ve.
- Positive in indeterminant and negative.
Another panel
Another panel for benign urothelium versus CIS:[7]
- CK20 +ve in deep cells (23/26 cases).
- Normal urothelium -- only the umbrella cells.
- Ki-67 ~50% of cells - deep and superficial.
- Normal ~10% of cells, confined to basal aspect.
- CD44 -ve.[8]
- Positive in indeterminant and negative.
Others
- AMACR +ve (80% and 50% of untreated and treated CIS respectively[8]).
Images
Sign out
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- UROTHELIAL CARCINOMA IN SITU.
- BENIGN MUSCULARIS PROPRIA PRESENT.
URINARY BLADDER, RANDOM BIOPSIES: - UROTHELIAL CARCINOMA IN SITU, SEE COMMENT. -- NO EVIDENCE OF LAMINA PROPRIA INVASION. - CHRONIC INFLAMMATION, MILD. - BENIGN MUSCULARIS PROPRIA PRESENT. COMMENT: A CK20 immunostain marks the full thickness of the urothelium in atypical areas. A p53 immunostain moderately marks up to 20% of atypical cells focally. A Ki-67 immunostain marks 20-50% of the cells in the atypical areas.
Micro
The sections show multiple fragments of urothelium with nuclear hyperchromasia, nuclear crowding, mild-to-moderate nuclear enlargement, several atypical mitoses, and lack of maturation to the surface. There is no evidence of invasion. Benign muscularis propria is present.
See also
References
- ↑ Nielsen, ME.; Smith, AB.; Meyer, AM.; Kuo, TM.; Tyree, S.; Kim, WY.; Milowsky, MI.; Pruthi, RS. et al. (Jan 2014). "Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006.". Cancer 120 (1): 86-95. doi:10.1002/cncr.28397. PMID 24122346.
- ↑ Hodges, KB.; Lopez-Beltran, A.; Davidson, DD.; Montironi, R.; Cheng, L. (Feb 2010). "Urothelial dysplasia and other flat lesions of the urinary bladder: clinicopathologic and molecular features.". Hum Pathol 41 (2): 155-62. doi:10.1016/j.humpath.2009.07.002. PMID 19762067.
- ↑ Nese, N.; Gupta, R.; Bui, MH.; Amin, MB. (Jan 2009). "Carcinoma in situ of the urinary bladder: review of clinicopathologic characteristics with an emphasis on aspects related to molecular diagnostic techniques and prognosis.". J Natl Compr Canc Netw 7 (1): 48-57. PMID 19176205.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 161. ISBN 978-0443066771.
- ↑ Amin, Mahul B. (2010). Diagnostic Pathology: Genitourinary (1st ed.). Amirsys. pp. 2-55. ISBN 978-1931884280.
- ↑ Amin MB, Epstein JI, Ulbright TM, et al. (August 2014). "Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference". Am. J. Surg. Pathol. 38 (8): 1017–22. doi:10.1097/PAS.0000000000000254. PMID 25025364.
- ↑ Yin, H.; He, Q.; Li, T.; Leong, AS. (Sep 2006). "Cytokeratin 20 and Ki-67 to distinguish carcinoma in situ from flat non-neoplastic urothelium.". Appl Immunohistochem Mol Morphol 14 (3): 260-5. PMID 16932015.
- ↑ 8.0 8.1 Aron, M.; Luthringer, DJ.; McKenney, JK.; Hansel, DE.; Westfall, DE.; Parakh, R.; Mohanty, SK.; Balzer, B. et al. (Dec 2013). "Utility of a triple antibody cocktail intraurothelial neoplasm-3 (IUN-3-CK20/CD44s/p53) and α-methylacyl-CoA racemase (AMACR) in the distinction of urothelial carcinoma in situ (CIS) and reactive urothelial atypia.". Am J Surg Pathol 37 (12): 1815-23. doi:10.1097/PAS.0000000000000114. PMID 24225842.