Difference between revisions of "Ependymoma"
Jump to navigation
Jump to search
Jensflorian (talk | contribs) (image + ref metaplasia in ependymoma) |
Jensflorian (talk | contribs) (→General: New WHO 2016 entity: Ependymoma, RELA-fusion positive) |
||
| Line 46: | Line 46: | ||
*May be associated with [[neurofibromatosis type 2]]. | *May be associated with [[neurofibromatosis type 2]]. | ||
There are | There are five main ependymal tumors:<ref name=Ref_WHOCNS_74>{{Ref WHOCNS|74}}</ref> | ||
#Ependymoma (not otherwise specified). | #Ependymoma (not otherwise specified). | ||
#*Other flavours:<ref>URL: [http://emedicine.medscape.com/article/1744030-overview http://emedicine.medscape.com/article/1744030-overview]. Accessed on: 17 January 2012.</ref> | #*Other flavours:<ref>URL: [http://emedicine.medscape.com/article/1744030-overview http://emedicine.medscape.com/article/1744030-overview]. Accessed on: 17 January 2012.</ref> | ||
#**Papillary ependymoma. | #**Papillary ependymoma. | ||
#**Clear cell ependymoma. | #**Clear cell ependymoma. | ||
#**Tanycytic ependymoma. | #**Tanycytic ependymoma. | ||
#Ependymoma, RELA fusion-positive.<ref>{{Cite journal | last1 = Parker | first1 = M. | last2 = Mohankumar | first2 = KM. | last3 = Punchihewa | first3 = C. | last4 = Weinlich | first4 = R. | last5 = Dalton | first5 = JD. | last6 = Li | first6 = Y. | last7 = Lee | first7 = R. | last8 = Tatevossian | first8 = RG. | last9 = Phoenix | first9 = TN. | title = C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. | journal = Nature | volume = 506 | issue = 7489 | pages = 451-5 | month = Feb | year = 2014 | doi = 10.1038/nature13109 | PMID = 24553141 }}</ref> | |||
#*L1CAM +ve / Nuclear NFkappaB +ve.<ref>{{Cite journal | last1 = Pietsch | first1 = T. | last2 = Wohlers | first2 = I. | last3 = Goschzik | first3 = T. | last4 = Dreschmann | first4 = V. | last5 = Denkhaus | first5 = D. | last6 = Dörner | first6 = E. | last7 = Rahmann | first7 = S. | last8 = Klein-Hitpass | first8 = L. | title = Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. | journal = Acta Neuropathol | volume = 127 | issue = 4 | pages = 609-11 | month = Apr | year = 2014 | doi = 10.1007/s00401-014-1264-4 | PMID = 24562983 }}</ref> | |||
#*Majority of supratentorial ependymoma in children. | |||
#Anaplastic ependymoma. | #Anaplastic ependymoma. | ||
#[[Myxopapillary ependymoma]]. | #[[Myxopapillary ependymoma]]. | ||
| Line 58: | Line 60: | ||
#[[Subependymoma]]. | #[[Subependymoma]]. | ||
#*Typically seen in IVth ventricle. | #*Typically seen in IVth ventricle. | ||
The designation ''Cellular ependymoma'' is depreceated. | |||
==Gross== | ==Gross== | ||
Revision as of 14:28, 18 May 2016
| Ependymoma | |
|---|---|
| Diagnosis in short | |
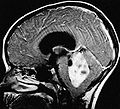
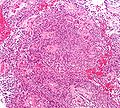
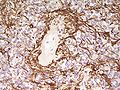
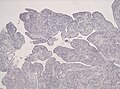
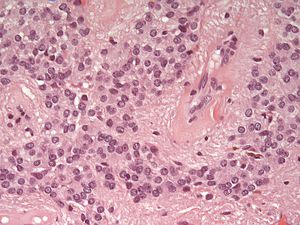 Ependymoma grade II WHO. H&E stain | |
|
| |
| LM | Perivascular pseudorosettes, ependymal rosettes |
| Subtypes | Tanycytic, Clear cell, Papillary, Cellular |
| LM DDx | Subependymoma, Glioblastoma, Pilocytic astrocytoma, Oligodendroglioma |
| IHC | GFAP +ve |
| Prognosis | intermediate to poor (WHO Grades II & III) |
Ependymoma is a neuropathology tumour.
- Myxopapillary ependymoma is dealt with separately in the myxopapillary ependymoma article.
- Subependymoma is dealt with separately in the Subependymoma article.
General
- Called the forgotten glial tumour.
Epidemiology:[1]
- Usual site:
- Adults: usually spinal cord.
- Children: usually posterior fossa.
- May be associated with neurofibromatosis type 2.
There are five main ependymal tumors:[2]
- Ependymoma (not otherwise specified).
- Other flavours:[3]
- Papillary ependymoma.
- Clear cell ependymoma.
- Tanycytic ependymoma.
- Other flavours:[3]
- Ependymoma, RELA fusion-positive.[4]
- L1CAM +ve / Nuclear NFkappaB +ve.[5]
- Majority of supratentorial ependymoma in children.
- Anaplastic ependymoma.
- Myxopapillary ependymoma.
- Classically at filum terminale.
- Subependymoma.
- Typically seen in IVth ventricle.
The designation Cellular ependymoma is depreceated.
Gross
- Usually discrete and enhancing.
- Ventricular location, but also within the spinal cord.
- Dissemination possible.
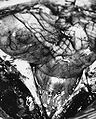
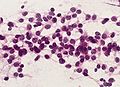
Microscopic
Classic ependymoma
Features:
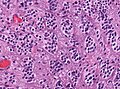
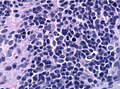
- Cells have a "tadpole-like" morphology.
- May also be described as ice cream cone-shaped.[6]
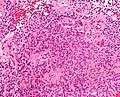
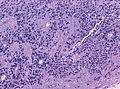
- Rosettes = circular nuclear free zones/cells arranged in a pseudoglandular fashion; comes in two flavours in ependymoma:
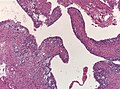
- Perivascular pseudorosettes = (tumour) cells arranged around a blood vessel; nuclei of cells distant from the blood vessel, i.e. rim of cytoplasm (from tumour cells) surround blood vessel (nucleus-free zone); more common than ependymal rosette... but less specific.
- Ependymal rosette (AKA true ependymal rosette) = rosette has an empty space at the centre - key feature.
- Nuclear features monotonous, i.e. "boring".[7]
- There is little variation in size, shape and staining.
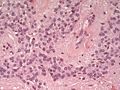
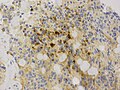
- Rare cases with cartilagineous metaplasia.[8]
DDx (classic ependymoma):
- Subependymoma.
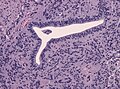
- Glioblastoma (GBM).
- Invasive border = GBM; circumscribed border of lesion = ependymoma.
- Pilocytic astrocytoma (Tanycytic ependymoma)
- Oligodendroglioma (Clear cell ependymoma))
Images
www:
- Ependymoma (flickr.com).
- Ependymoma - ependymal rosettes (ajnr.org).
- Anaplastic ependymoma - case 1 (upmc.edu).
- Anaplastic ependymoma - case 2 (upmc.edu).
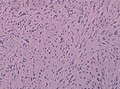
Tanycytic ependymoma must not confused with pilocytic astrocytoma. (WC/jensflorian)
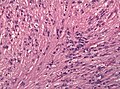
Clear cell ependymoma may mimic oligodendroglioma. (WC/jensflorian)
Grading
Easy:
- Subependymoma = WHO grade I.
- Myxopapillary ependymoma = WHO grade I.
Not-so-easy:
- Classic ependymoma = WHO grade II.
- Anaplastic ependymoma = WHO grade III.
Grade II vs. Grade III:
- Cellular density.
- Mitoses.
- Necrosis.
- Microvascular proliferation.
Notes:
- Many tumours fall between grade II and grade III. These are called "indeterminate" by many.
IHC
- Reticulin.
- GFAP+ve.
- MIB1.
- EMA (dots and rings).
Molecular
Two distinct molecular subgroups exist in the posterior fossa:[9]
- Group A ependymomas:
- typically found in children.
- laterally.
- relatively unfavorable clinical outcome.
- CpG island methylator phenotype.[10]
- Group B ependymomas:
- typically adults.
- midline.
- relatively favorable clinical outcomes.
- gene expression profiles similar to that of spinal cord ependymomas.
- increased Chromosomal 1q gains. [11]
Supratentorial ependymomas have also a distinct profile:
- 70 % of these ependymomas have recurrent gene fusions involving RELA and C11orf95[12]
- EPHB2 amplifications and CDKN2A deletions in a subset of these tumors[13]
Note: Molecular subgroups have no treatment implications (at the moment).
See also
References
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1334. ISBN 978-1416031215.
- ↑ The International Agency for Research on Cancer (Editors: Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.) (2007). Pathology and Genetics of Tumours of Tumors of the Central Nervous System (IARC WHO Classification of Tumours) (4th ed.). Lyon: World Health Organization. pp. 74. doi:10.1007/s00401-007-0243-4. ISBN 978-9283224303.
- ↑ URL: http://emedicine.medscape.com/article/1744030-overview. Accessed on: 17 January 2012.
- ↑ Parker, M.; Mohankumar, KM.; Punchihewa, C.; Weinlich, R.; Dalton, JD.; Li, Y.; Lee, R.; Tatevossian, RG. et al. (Feb 2014). "C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma.". Nature 506 (7489): 451-5. doi:10.1038/nature13109. PMID 24553141.
- ↑ Pietsch, T.; Wohlers, I.; Goschzik, T.; Dreschmann, V.; Denkhaus, D.; Dörner, E.; Rahmann, S.; Klein-Hitpass, L. (Apr 2014). "Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway.". Acta Neuropathol 127 (4): 609-11. doi:10.1007/s00401-014-1264-4. PMID 24562983.
- ↑ http://www.pathology.vcu.edu/WirSelfInst/tumor-2.html
- ↑ MUN. 6 Oct 2009.
- ↑ Wang, X.; Zhang, S.; Ye, Y.; Chen, Y.; Liu, X. (Jul 2012). "Ependymoma with cartilaginous metaplasia might have more aggressive behavior: a case report and literature review.". Brain Tumor Pathol 29 (3): 172-6. doi:10.1007/s10014-011-0079-4. PMID 22228122.
- ↑ Witt, H.; Mack, SC.; Ryzhova, M.; Bender, S.; Sill, M.; Isserlin, R.; Benner, A.; Hielscher, T. et al. (Aug 2011). "Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma.". Cancer Cell 20 (2): 143-57. doi:10.1016/j.ccr.2011.07.007. PMID 21840481.
- ↑ Mack, SC.; Witt, H.; Piro, RM.; Gu, L.; Zuyderduyn, S.; Stütz, AM.; Wang, X.; Gallo, M. et al. (Feb 2014). "Epigenomic alterations define lethal CIMP-positive ependymomas of infancy.". Nature 506 (7489): 445-50. doi:10.1038/nature13108. PMID 24553142.
- ↑ Korshunov, A.; Witt, H.; Hielscher, T.; Benner, A.; Remke, M.; Ryzhova, M.; Milde, T.; Bender, S. et al. (Jul 2010). "Molecular staging of intracranial ependymoma in children and adults.". J Clin Oncol 28 (19): 3182-90. doi:10.1200/JCO.2009.27.3359. PMID 20516456.
- ↑ Parker, M.; Mohankumar, KM.; Punchihewa, C.; Weinlich, R.; Dalton, JD.; Li, Y.; Lee, R.; Tatevossian, RG. et al. (Feb 2014). "C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma.". Nature 506 (7489): 451-5. doi:10.1038/nature13109. PMID 24553141.
- ↑ Philip-Hollingsworth, S.; Hollingsworth, RI.; Dazzo, FB. (Jan 1989). "Host-range related structural features of the acidic extracellular polysaccharides of Rhizobium trifolii and Rhizobium leguminosarum.". J Biol Chem 264 (3): 1461-6. PMID 2912966.