Difference between revisions of "Atypical ductal hyperplasia"
Jump to navigation
Jump to search
(→IHC) |
(→Images) |
||
| Line 32: | Line 32: | ||
<gallery> | <gallery> | ||
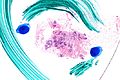
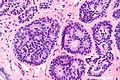
Image:Atypical_ductal_hyperplasia_-_very_low_mag.jpg|ADH. Very low mag. (WC/Nephron) | Image:Atypical_ductal_hyperplasia_-_very_low_mag.jpg|ADH. Very low mag. (WC/Nephron) | ||
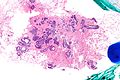
Image:Atypical_ductal_hyperplasia_-_low_mag.jpg|ADH - low mag. (WC/Nephron) | |||
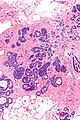
Image:Atypical_ductal_hyperplasia_-_intermed_mag.jpg|ADH - intermed. mag. (WC/Nephron) | |||
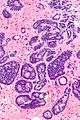
Image:Atypical_ductal_hyperplasia_-_high_mag.jpg|ADH - high mag. (WC/Nephron) | Image:Atypical_ductal_hyperplasia_-_high_mag.jpg|ADH - high mag. (WC/Nephron) | ||
Image:Atypical_ductal_hyperplasia_-_very_high_mag.jpg|ADH - very high mag. (WC/Nephron) | |||
</gallery> | </gallery> | ||
==IHC== | ==IHC== | ||
*CK5 <20% +ve. | *CK5 <20% +ve. | ||
Revision as of 15:30, 28 April 2016
Atypical ductal hyperplasia, abbreviated ADH, a benign breast lesion associated with an increased risk of malignancy.
General
- Molecular studies have shown it is the same thing as low-grade DCIS; thus, some have called for abolition of the term.[1]
- ADH is considered an indication for a lumpectomy.[2]
Epidemiology:
- Relative risk of breast cancer, based on a median follow-up of 8 years, in a case control study of US registered nurses, is 3.7.[5]
Microscopic
Features:
- Cytologic and architectural feature of low-grade DCIS.
- Cell spacing ~ equal.
- Lumina round.
- Architecture - classically cribriform or solid; may be micropapillary or papillary.
- Small nuclei.
- Small indistinct nucleoli.
- Limited extent (diagnostic size cutoffs) - either:[6]
- < Two complete ducts.
- < 2 mm. ‡
DDx:
- Low-grade DCIS.
- Florid epithelial hyperplasia of the usual type (FEHUT).
Notes:
- High-grade DCIS is not in the DDx of ADH.
- ‡ 3 mm is used in papillary lesions.[citation needed]
Images
IHC
- CK5 <20% +ve.
- ER +ve - diffusely.
- Heterogenous in FEHUT.
See also
References
- ↑ Ghofrani, M.; Tapia, B.; Tavassoli, FA. (Dec 2006). "Discrepancies in the diagnosis of intraductal proliferative lesions of the breast and its management implications: results of a multinational survey.". Virchows Arch 449 (6): 609-16. doi:10.1007/s00428-006-0245-y. PMID 17058097.
- ↑ Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP (May 1995). "Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy". AJR Am J Roentgenol 164 (5): 1111–3. PMID 7717215. http://www.ajronline.org/cgi/pmidlookup?view=long&pmid=7717215.
- ↑ Deshaies, I.; Provencher, L.; Jacob, S.; Côté, G.; Robert, J.; Desbiens, C.; Poirier, B.; Hogue, JC. et al. (Feb 2011). "Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy.". Breast 20 (1): 50-5. doi:10.1016/j.breast.2010.06.004. PMID 20619647.
- ↑ Margenthaler, JA.; Duke, D.; Monsees, BS.; Barton, PT.; Clark, C.; Dietz, JR. (Oct 2006). "Correlation between core biopsy and excisional biopsy in breast high-risk lesions.". Am J Surg 192 (4): 534-7. doi:10.1016/j.amjsurg.2006.06.003. PMID 16978969.
- ↑ London, SJ.; Connolly, JL.; Schnitt, SJ.; Colditz, GA. (Feb 1992). "A prospective study of benign breast disease and the risk of breast cancer.". JAMA 267 (7): 941-4. PMID 1734106.
- ↑ Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 258. ISBN 978-0470519035.