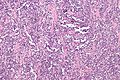
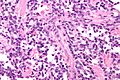
Rhabdomyosarcoma
Jump to navigation
Jump to search
The printable version is no longer supported and may have rendering errors. Please update your browser bookmarks and please use the default browser print function instead.
| Rhabdomyosarcoma | |
|---|---|
| Diagnosis in short | |
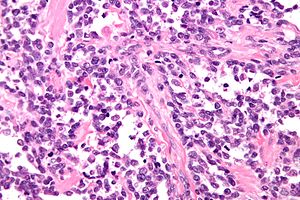 Alveolar rhabdomyosarcoma. H&E stain. | |
|
| |
| LM | +/-rhabdomyoblasts (eccentric nucleus, moderate amount of intensly eosinophilic cytoplasm, striations - not common); alveolar RMS: alveolus-like pattern (classic); embryonal RMS: small round cell tumour |
| Subtypes | embryonal (spindle cell subtype, botryoid), alveolar (translocation-positive, translocation-negative), undifferentiated |
| LM DDx | small round cell tumours - esp. small cell carcinoma and (large cell) lymphomas |
| IHC | desmin (best marker) +ve, actin +ve, myogenin +ve, CD56 +ve (common), synaptophysin -ve/+ve, chromogranin -ve/+ve, cytokeratins -ve/+ve |
| EM | sarcomeric like structures - typically in U-shaped cells |
| Molecular | alveolar RMS (~85% of cases): t(2,13) PAX3/FKHR fusion gene or t(1,13) PAX7/FKHR fusion gene |
| Site | soft tissue - skeletal muscle site (alveolar RMS), non-skeletal muscle site (embryonal RMS) |
|
| |
| Syndromes | DICER1 syndrome for embryonal rhabdomyosarcoma |
|
| |
| Clinical history | alveolar RMS: young adult or adolescent; embryonal RMS: typically <10 years old |
| Prevalence | not common |
| Clin. DDx | other soft tissue tumours |
Rhabdomyosarcoma, often abbreviated RMS, is a malignant tumour of skeletal muscle.
General
- Most common paediatric sarcoma.
- Classically in the head and neck region.[1]
- Most common sarcoma in Li-Fraumeni syndrome.[2]
- ~6% of all childhood cancer.
Classification
Histologic
- Alveolar rhabdomyosarcoma.
- Usually young adults/adolescents.
- Early mets common.
- Usually arises in regions with skeletal muscle.
- Embryonal rhabdomyosarcoma.
- Usual <10 years old.
- Typically locally invasive.
- Usually arises in regions without skeletal muscle.
Less common types:[3]
- Undifferentiated rhabdomyosarcoma.
- Botryoid - may be considered a subtype of embryonal RMS.
- Spindle cell - may be considered a subtype of embryonal RMS.
Notes:
- How to remember the special types BUS: botryoid, undifferentiated, spindle.
- The above is the international classification. Several classification of RMS exist - see: Classifications of Rhabdomyosarcoma.[4]
Molecular and histologic
- Translocation-positive alveolar RMS.
- Translocation-negative alveolar RMS.
- Embryonal RMS.
Notes:
- Translocation-negative alveolar RMS shares gene expression profiling characteristics with embryonal RMS -- suggesting these can be grouped together.
Gross
Sarcoma botryoides (embryonal RMS) - distinctive appearance:
- Grapes on the vine-like clusters.
- Found in urinary bladder, vagina.
Image:
Microscopic
Alveolar rhabdomyosarcoma
Features:[2]
- Alveolus-like pattern -- key low-power feature.
- Fibrous septae lined by tumour cells.
- Cells may "fall-off" the septa, i.e. be detached/scattered in the alveolus-like space.
- Space between fibrous sepate may be filled with tumour = solid variant of alveolar rhabdomyosarcoma.
- Fibrous septae lined by tumour cells.
- Rhabdomyoblasts - essentially diagnostic.
- Eccentric nucleus.
- Moderate amount of intensly eosinophilic cytoplasm.
- Striations -- if you're really lucky; these are not common.
Other features:
- Nuclear pleomorphism - common.
- Mitoses - common.
Notes:
- Well-differentiated rhabdomyoblasts are uncommon in alveolar RMS.
DDx:
- Alveolar soft part sarcoma.
- Skeletal muscle regeneration.[5]
Images
www:
Embryonal rhabdomyosarcoma
Features:[2]
- Randomly arranged small cells.
- Myxoid matrix.
- Strap cells:
- Tadpole-like morphology.
- Rhabdomyoblasts - essentially diagnostic.
- Eccentric nucleus.
- Moderate amount of intensly eosinophilic cytoplasm.
- Striations -- if you're really lucky; these are not common.
DDx:
Images:
Subtypes of embryonal RMS
There are two common subtypes of embryonal RMS. Both of them have a better prognosis that embryonal RMS not otherwise specified (NOS).
Common subtypes:
- Botryoid subtype (AKA sarcoma botryoides):
- Gross: Grape-like morphology.
- Microscopic: Non-proliferating layer deep to the surface ("Cambium layer").
- Spindle cell subtype.
- General: may mimic leiomyosarcoma (complete with vesicular pattern) -- which is not common in the pediatric population.
- Microscopic: vesicular growth pattern, spindle cells.
Notes:
- Cambium layer = cellular region deep to epithelial component.[7]
- Can be thought of as the opposite of a "Grenz zone" -- which is a paucicellular zone between tumour and epithelium.
Anaplasia
Criteria:
- Hyperchromatic nuclei with size variation greater or equal to 3x.
- Multipolar (atypical) mitotic figures.
Subclassification:
- Focal - a few cells.
- Diffuse - cluster or sheets of anaplasia.
Notes:
- Not subtle - can identify at low power.
- Seen in 10-15% of RMS.
- More common in older individuals.
- Poorer prognosis in embryonal RMS.
- No change in prognosis in alveolar RMS.
IHC
Panel of muscle markers -- DAM:
- Desmin (best marker).
- Actin.
- Myogenin.
For head and neck RMS:[8]
- CD56 +ve.
- Synaptophysin -ve/+ve (seen in 12 of 37 cases[8]).
- Chromogranin A -ve/+ve (seen in 8 of 36 cases[8]).
- Wide-spectrum cytokeratin -ve/+ve.
- CAM5.2 -ve/+ve.
For urinary bladder RMS in adults:
- Myogenin +ve.
- Desmin +ve.
- Keratins -ve.[9]
- Keratin positive tumours are considered rhabdomyosarcomatous sarcomatoid carcinoma or sarcomatoid carcinoma with rhabdomyosarcomatous differentiation.
Subtyping via IHC
PST proposes[2] the following (presumably based on Makawitz et al.[10]):
| IHC | Translocation positive alveolar RMS |
Embryonal RMS | Translocation negative alveolar RMS |
| myogenin | +ve -- diffuse | +ve -- focal | +ve -- diffuse |
| EGFR | -ve | +ve | -ve |
| P-cadherin | +ve | -ve | -ve |
| IGF2 | -ve | +ve | +ve |
A paper by Wachtel at al.[11] proposes the use of:
- AP2beta and P-cadherin +ve in translocation positive alveolar RMS, and
- EGFR and fibrillin-2 +ve in embryonal RMS and translocation negative alveolar RMS.
Electron microscopy
Features:
- Sarcomeric like structures - usually in "bent" cells; cells that are U-shaped.
Molecular diagnostics
Alveolar rhabdomyosarcoma
Common translocations (~85% of cases):
- t(1,13).
- PAX7/FKHR fusion gene.
- Seen in approx. 15% of cases.
- t(2,13).[12]
- PAX3/FKHR fusion gene.
- Seen in approx. 70% of cases.
Notes:
- t(1,13) vs. t(2,13) -- t(1,13) usually: younger age, extremity lesion, localized disease, better survival.
- Several uncommon translocations exist.
- Important for urinary bladder lesions in adults: the presence of a translocation is more-or-less required for the diagnosis of RMS.[9]
- It is suggested that keratin negative tumours without molecular testing to corroborate the impression of RMS be referred to as rhabdomyomatous tumours.[9]
Embryonal rhabdomyosarcoma
- Chromosome 11p loss of heterozygosity.[13]
Note:
- Not used for diagnosis.
See also
References
- ↑ Rosenthal, TC.; Kraybill, W. (Aug 1999). "Soft tissue sarcomas: integrating primary care recognition with tertiary care center treatment.". Am Fam Physician 60 (2): 567-72. PMID 10465231.
- ↑ 2.0 2.1 2.2 2.3 Thorner, Paul S. 14 February 2011.
- ↑ Hicks, J.; Flaitz, C. (Jul 2002). "Rhabdomyosarcoma of the head and neck in children.". Oral Oncol 38 (5): 450-9. PMID 12110339.
- ↑ Parham, DM. (May 2001). "Pathologic classification of rhabdomyosarcomas and correlations with molecular studies.". Mod Pathol 14 (5): 506-14. doi:10.1038/modpathol.3880339. PMID 11353062.
- ↑ Guillou, L.; Coquet, M.; Chaubert, P.; Coindre, JM. (Aug 1998). "Skeletal muscle regeneration mimicking rhabdomyosarcoma: a potential diagnostic pitfall.". Histopathology 33 (2): 136-44. PMID 9762546.
- ↑ Chen, S.; Wang, S.; Gao, J.; Zhang, S. (May 2010). "[Pleuropulmonary blastoma: a clinicopathological analysis].". Zhongguo Fei Ai Za Zhi 13 (5): 550-3. doi:10.3779/j.issn.1009-3419.2010.05.31. PMID 20677658.
- ↑ URL: http://www.medilexicon.com/medicaldictionary.php?t=48297. Accessed on: 9 August 2011.
- ↑ 8.0 8.1 8.2 Bahrami, A.; Gown, AM.; Baird, GS.; Hicks, MJ.; Folpe, AL. (Jul 2008). "Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall.". Mod Pathol 21 (7): 795-806. doi:10.1038/modpathol.2008.86. PMID 18487991.
- ↑ 9.0 9.1 9.2 Bing, Z.; Zhang, PJ. (2011). "Adult urinary bladder tumors with rhabdomyosarcomatous differentiation: clinical, pathological and immunohistochemical studies.". Diagn Pathol 6: 66. doi:10.1186/1746-1596-6-66. PMID 21762516.
- ↑ Makawita S, Ho M, Durbin AD, Thorner PS, Malkin D, Somers GR (2009). "Expression of insulin-like growth factor pathway proteins in rhabdomyosarcoma: IGF-2 expression is associated with translocation-negative tumors". Pediatr. Dev. Pathol. 12 (2): 127–35. doi:10.2350/08-05-0477.1. PMID 18788888.
- ↑ Wachtel M, Runge T, Leuschner I, et al. (February 2006). "Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry". J. Clin. Oncol. 24 (5): 816–22. doi:10.1200/JCO.2005.03.4934. PMID 16391296.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/606597. Accessed on: 18 August 2010.
- ↑ Gallego Melcón, S.; Sánchez de Toledo Codina, J. (Jul 2007). "Molecular biology of rhabdomyosarcoma.". Clin Transl Oncol 9 (7): 415-9. PMID 17652054.