Difference between revisions of "Pancreas"
(→Pancreatic acinar metaplasia: +image) |
|||
| Line 165: | Line 165: | ||
*[[Pancreatic ductal adenocarcinoma|Ductal adenocarcinoma]]. | *[[Pancreatic ductal adenocarcinoma|Ductal adenocarcinoma]]. | ||
**Mucinous noncystic carcinoma. | **Mucinous noncystic carcinoma. | ||
**Signet ring cell carcinoma. | **[[Signet ring cell carcinoma]]. | ||
**Adenosquamous carcinoma. | **[[Adenosquamous carcinoma]]. | ||
**Undifferentiated carcinoma. | **Undifferentiated carcinoma. | ||
**Undifferentiated carcinoma with osteoclast-like giant cells. | **Undifferentiated carcinoma with osteoclast-like giant cells. | ||
Revision as of 07:48, 16 July 2013
The pancreas hangs-out in the upper abdomen. It occasionally is afflicited by cancers, the most common of which is very fatal.
Pancreatic cytopathology is dealt with in the gastrointestinal cytopathology article.
A general introduction to gastrointestinal pathology is in the gastrointestinal pathology article.
Introduction
Normal anatomy
Divided into three portions: head, body & tail:[1]
- Head:
- Includes unicate process.
- Extends to the left edge of the superior mesenteric vein (SMV) - by definition.
- All of the SMV is with the head.
- Body:
- Right edge of the superior mesenteric vein to the left edge of aorta - by definition.
- All of the aorta is with the body.
- Right edge of the superior mesenteric vein to the left edge of aorta - by definition.
- Tail:
- Remainder of pancreas.
Pancreatic surgeries
Common pancreatic surgeries include:
- Whipple procedure (AKA pancreaticoduodenal resection) - includes duodenum +/- stomach.
- Distal pancreatectomy.
- Removal of tail +/- body.
- Specimen usually comes with a spleen.
- Typically done form islet cell tumours.
- Total pancreatectomy.
- Specimen usually comes with a spleen.
Whipple procedure
- Proximal mucosal margin (stomach or duodenum).
- Distal mucosal margin (duodenum or jejunum).
- Bile duct margin.
- Pancreatic retroperitoneal (uncinate process) margin.
- At SB done on edge (not en face).
- Pancreatic neck transection margin (AKA distal pancreatic resection margin);[3] usu. en face and in toto.[4]
- Sometimes superior mesenteric vein (SMV).
- Rarely superior mesenteric artery (SMA) margin.
General classification of pancreatic tumours
- Metstatses.
- Most common = renal cell carcinoma.
- Primary.
- Endocrine.
- Usually small as hormonally active.
- Exocrine.
- Endocrine.
Pancreas neoplasms in a table
| Type | Key feature | Subtypes | Image | IHC | Detailed microscopic | Usual location | Other | DDx |
|---|---|---|---|---|---|---|---|---|
| Serous tumours | cuboidal cells, clear cytoplasm | cystadenoma, borderline t., cystadenocarcinoma | [1], (WC), (WC) | IHC? | cuboidal cells, clear cytoplasm, central nucleus | body or tail | cystadenoma may be assoc. with von Hippel-Lindau syndrome | clear cell RCC, oligomucinous mucinous tumours |
| Intraductal papillary mucinous tumour (IPMT) | mucin, no ovarian-like stroma | clear cell variant | (wjso.com), (upmc.edu) | IHC? | papillae, tall columnar mucin-producing cells | head | - | mucious neoplasms (other pancreatic, duodenal), intra-ampullary papillary-tubular neoplasm (see ampullary carcinoma) |
| Mucinous tumour | mucin, ovarian-like stroma | cystadenoma, borderline t., cystadenocarcinoma | (WC), (WC) | IHC? | tall columnar mucin-producing cells, ovarian-like stroma | body or tail | - | IPMT, metastatic mucinous tumours |
| Solid pseudopapillary tumour |
eosinophilic intracytoplasmic globules | clear cell variant (cytoplasm clear) | (WC), (bmj.com) | beta-catenin +ve, E-cadherin +ve, synaptophysin +ve, chromogranin -ve |
sheets of cells, focally loosely cohesive, eosinophilic cytoplasm, uniform nuclei with grooves | none (head, body or tail) | usu. younger women | ductal adenocarcinoma, neuroendocrine tumours |
| Ductal adenocarcinoma | irregular shaped glands, cytologic atypia | mucinous, spindle cell, mixed ductal-endocrine | (WC), (WC) | IHC? | glands, sheets, single cells, nuc. atypia, +/-mitoses, +/-necrosis | head | arises from the precursor PanIN | ampullary carcinoma, chronic pancreatitis |
| Pancreatoblastoma | squamoid nests, whorling | - | (nature.com) | CK7 (acinar comp.), CK8, CK18, CK19 | squamoid nests of cells, whorling, nested growth, +/-keratinization | none | usu. paediatric population | acinar cell carcinoma |
| Acinar cell carcinoma | acinar arch. | - | (WC), (histopathology-india.net) | trypsin, lipase | nests or trabeculae, nucleolus, mod. basophilic granular cytoplasm | head (slight predilection) | - | pancreatoblastoma |
| Undifferentiated carcinoma with osteoclast-like giant cells | giant cells | - | Image? | IHC? | giant cells, usu. with AIS or inv. ductal adenocarcinoma | head | - | anaplastic carcinoma |
| Chronic pancreatitis | fibrosis, loss of acinar tissue, preservation of lobular arch. | - | [2] | IHC? | loss of acinar tissue with preservation of islets, fibrosis | ? | not a neoplasm, included here as it is in the (clinical) DDx | ductal adenocarcinoma |
WHO classification
Benign epithelial:
Borderline epithelial:
- Mucinous cystic neoplasm with moderate dysplasia.
- Intraductal papillary mucinous neoplasm with moderate dysplasia.
- Solid pseudopapillary neoplasm
Malignant epithelial:
- Ductal adenocarcinoma.
- Mucinous noncystic carcinoma.
- Signet ring cell carcinoma.
- Adenosquamous carcinoma.
- Undifferentiated carcinoma.
- Undifferentiated carcinoma with osteoclast-like giant cells.
- Mixed ductal-endocrine carcinoma.
- Serous cystadenocarcinoma.
- Mucinous cystadenocarcinoma.
- Invasive.
- Noninvasive.
- Intraductal papillary mucinous carcinoma.
- Invasive.
- Noninvasive.
- Acinar cell carcinoma.
- Pancreatoblastoma.
- Solid pseudopapillary carcinoma.
Soft tissue tumours:
- See soft tissue lesions.
Ectopic pancreatic tissue
It comes in two flavours:[5]
- Pancreatic ectopia.
- Pancreatic (acinar) metaplasia.
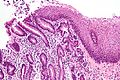
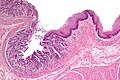
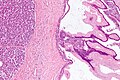
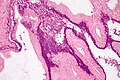
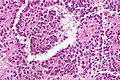
Pancreatic acinar metaplasia
General
- Common in the GI tract.
- Found in ~ 20% of eosphageal biopsies above the GEJ.[7]
Gross
- May be a single lesion or a cluster of lesions.[6]
Microscopic
Features:
- Pancreatic acini - only.
- Intensely eosinophilic cytoplasm.
Negatives:
- No pancreatic ducts.
- No islets of Langerhans (pancreatic islets).
Images
IHC
Features:[8]
- Trypase +ve.
- Lipase +ve.
Pancreatic ectopia
General
- May be confused with something pathologic.
Microscopic
Features:
- Consists of pancreatic acini and pancreatic ducts.
- +/-Islets of Langerhans.
Inflammatory
Pancreatitis
Classification
Etiology
Mnemonic I GET SMASHED:
- Idiopathic.
- Gallstones ~45%.
- Ethanol ~35%.
- Tumours (pancreas, ampulla).
- Scorpion bites, snake bites.
- Microbial - mumps (paramyxovirus), Epstein-Barr virus (EBV), cytomegalovirus (CMV), mycoplasma.
- Autoimmune - Crohn's disease, polyarteritis nodosa (PAN), systemic lupus erythematosus (SLE).
- Surgery/trauma, e.g. ERCP, motor vehicle collision.
- Hypercalcemia, hyperlipidemia/hypertriglyceridemia, hypothermia.
- Emboli, e.g. post-CABG.
- Drugs - SAND = steroids & sulfonamides, azathioprine, NSAIDs, diuretics, such as furosemide.
Acute pancreatitis
General
- Rarely comes to pathology.
- Usually diagnosed by abdominal CT, blood work (amylase, lipase).
Microscopic
Features:[9]
- Loss of acini.
- Neutrophils.
- Hemorrhage.
- +/-Loss of pancreatic islets.
Chronic pancreatitis
General
- May be confused with ductal adenocarcinoma radiologically... and pathologically.
- Often due to ethanol abuse.
Complications:[10]
- Malabsorption.
- Diabetes mellitus.
- Pseudocysts.
Autoimmune pancreatitis
Histologic subtypes of autoimmune pancreatitis:[11]
- Lymphoplasmacytic sclerosing pancreatitis (LPSP).
- Typically IgG4 positive -- one of the IgG4-related systemic diseases.
- Approximately 80% of cases.[12]
- Idiopathic duct-centric chronic pancreatitis (IDCP).
- Typically IgG4 negative.
- Approximately 20% of cases.
Lymphoplasmacytic sclerosing pancreatitis
General:
- Serum IgG4 +ve.[14]
Microscopic:
- Lymphoplasmacytic infiltrate.
IHC:
- Plasma cells IgG4 +ve.
Radiology
Plain film findings:
- Calcifications.
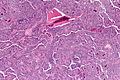
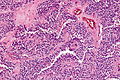
Microscopic
Features of chronic pancreatitis:[15]
- Preservation of lobular architecture - evenly spaced ductal units.
- Uniformly sized ductal elements.
- Smooth ductal contours.
- Ducts surrounded by acini or islets.
- Islets usu. preserved better than acini.[16]
- Intraluminal mucoprotein plugs.
Images:
Adenocarcinoma versus pancreatitis
This contrasts with the features of adenocarcinoma:[15]
- Ductal architecture:
- Random distribution of ductal structures.
- Irregular ductal contours.
- "Naked ducts in fat"; ducts without surrounding pancreatic elements or fibrous tissue.
- Ducts adjacent to arterioles.
- Nuclear atypia:
- Enlargement (>3 times the size of a lymphocyte).
- Pleomorphism.
- Distinct nucleoli.
- Hyperchromatic raisinoid nucleoli.
- Generally assoc. with malignancy:
- Perineural and vascular invasion (rare).
- Mitosis.
- Necrotic cellular debris (intraluminal).
Notes:
- Memory device: give 'em a fair chance at a benign diagnosis. Features suggestive of malignant:
- Fat, adjacent to.
- Arteriole, adjacent to.
- Irregular ducts.
- Random distribution of ducts/non-lobular arrangement.
IHC
- IgG4 +ve plasma cells -- IgG4 sclerosing disease.
Positive in pancreatic carcinoma:[17]
- p53.
- Mesothelin.
Cystic lesions - overview
General
- True cystic lesions are uncommon.
- A true cystic lesion: must have an epithelial lining.
- Only 10% of cystic lesions are true cystic lesions, i.e. 90% of cystic lesions are really pseudocysts.
- A true cystic lesion: must have an epithelial lining.
- It is hard to differentiate pseudocysts & cysts.
Cystic tumours - clinical
General:
- Usually diagnosed by imaging (CT/MRI, ERCP, Endoscopic ultrasound).
- 50% incidental finding.
- Vague symptoms
- Abdominal mass.
- Weight loss.
- Jaundice.
- Usually favourable prognosis - mostly benign.
Most important cystic lesions
- Serous.
- Mucinous.
- Ovarian-like stroma.
- Solid pseudopapillay tumours.
- Intraductal papillary mucinous tumour (IPMT).
- No ovarian-like stroma.
Mnemonic SIMS: Serous, IPMT, Mucinous, Solid pseudopapillary tumour.
Useful stains
- PAS-D.
Mucinous vs. IMPT
IMPT:
- No ovarian-like stroma.
- Usually has total pancreatectomy.
Cystic tumours of the pancreas
Khalifa's table of cystic tumours:
| Tumour | Usual sex | Age (years) | Usual site | Typical size (cm) |
Gross pathology |
|---|---|---|---|---|---|
| Serous microcystic adenoma |
female | 66 | body & tail | 11 | (joplink.net[18], (jhmi.edu)[19] |
| Intraductal papillary mucinous tumour (IPMT) |
male | 62 | head | 4 | (jhmi.edu)[19] |
| Mucinous tumour | female | 49 | body & tail | 10 | (rsna.org) |
| Solid pseudopapillary tumour |
female | 35 | any | 7.5 | (ajronline.org), (flickr.com/humpath) |
Cystic lesions
Serous tumours - overview
General
- Cell of origin: intralobular duct cells (ductular cells).
- Glycogen rich - but do not produce mucin.
Subclassication
- Serous microcystic adenoma (AKA serous cystadenoma[20]).
- Many small cysts.
- Serous oligocystic adenoma.
- Large cysts.
- Serous cystadenocarcinoma - very rare.[21]
Note:
- If one mucin +ve cell, tumour = a mucinous tumour.
Serous cystadenoma of the pancreas
General
- 1-2% of all exocrine pancreatic tumours.
- Female > male.
- Mean age 66 years.
- Truly benign with no malignant potenial.
- May be part of von Hippel-Lindau syndrome.
Management:
- Observe or resect.
Gross
Features:
- Classically has a characteristic central scar.[22]
- Bosulated surface.
- Lobulated.
- No (macroscopic) cysts apparent on gross.
- Location: 50-70% occur in the body and tail.
- Size: average size 11 cm.
Radiologic appearance:
- Honey comb-like appearance.
- Well demarcated border - may be described as a "coin lesion".
Image:
Microscopic
Features:
- Cystic spaces lined by cuboidal cells.
- Glycogen rich.
- Cilia. (???)
DDx:
- Renal cell carcinoma.
- Lymphangioma.
- Hemangioma.
- Oligocystic mucinous cystic tumors and pseudocysts.
- Have mucin; PAS-D could be used to demonstrate its presence.
Notes:
- Serous adenoma may coexist with aggressive tumours.
Images
Stains
- PAS +ve.
- PASD -ve.
Mucinous cystic neoplasms of the pancreas
- Gastro-entero-pancreatic cell differentiation with hypercellular ovarian-type stroma.
- Stroma --> cellular.
- 2-2.5% of all exocrine pancreatic tumours.
- Almost exclusively in women.
- Mean age - 49 years.
- >80% in body and tail.
- Average size ~10 cm.
Note:
- Looks different than serous tumour.
Subclassification
- Mucinous cystadenoma.
- Borderline mucinous cystic tumour.
- Mucinous cystadenocarcinoma.
Borderline vs. Carcinoma
- Few mitoses in borderline.
Radiology
- Mucinous tumours: multilocular.
- Generally larger than serous.
- Often partially solid and cystic.
- Often calcified.
- Calcification rare in serous.
- Usually tail & body.
Microscopic
Mucinous cystadenoma
Features:[24]
- Simple tall columnar epithelium with large mucin vacuole on apical aspect.
- "Ovarian-type stroma" under epithelium.
- Ovarin-type stroma: high density of small (non-wavy) spindle cells with eosinophilic cytoplasm.
Notes:
- Appearance similar to mucinous cystadenoma in the ovary.
- Mucin stains +ve (intracytoplasmic).
Images
www:
Borderline mucinous cystic tumour
Features:
- May have finger like projections.
- Pseudostratification of epithelium.
Notes:
- Surgery does not change based on diagnosis on frozen section.
- Only question is "Is the margin clear?".
- Borderline tumours are rare.
Carcinoma
- Cells floating in mucin.
Mucinous tumour versus pseudocyst
| Finding | Mucinous tumour | Pseudocyst |
|---|---|---|
| Amylase & lipase | low | high |
| Viscosity | high | low |
| CEA, CA125 | high | low |
Prognosis:
- Benign looking tumours have the potential to transform into carcinoma.
- No report of assoc. pseudomyxoma peritonei.
- US boards question -- it is an exception ... others one cause it.
- Prognosis of m. cystadenocarcinoma is slightly better than that of ductal adenocarcinoma.
Intraductal papillary mucinous tumour
- Abbreviated IPMT.
- AKA intraductal papillary mucinous neoplasm, abbreviated IPMN.
General
- Morphologically and biologically distinct from ductal adenocarcinoma, mucinous cystic tumour and ductal papillary hyperplasia.
- Prognosis:
Clinical:
- Patient usually not jaundiced... as no obstruction.
- Often diabetes... as pancreas is destroyed.
- Patients may get a total pancreatectomy - as the disease is often multifocal.
Epidemiology
- ~1% of all exocrine pancreatic tumours.
- More common in males.
- Mean age at presentation 62 years.
- 60-80% occur in the head of the pancreas.
- Average size 4 cm.
Gross
- May be patchy/multifocal.
- Multiple cystic spaces.
Microscopic
Features:
- Pancreatic duct lining cells jut into the duct lumen - papillomatous growth pattern.
- Cytology:
- Cell enlargement.
- Increased mucin production.
- Nuclear changes:
- Increased NC ratio.
- Nuclear crowding and pleomorphism.
- Mitotic activity.
- Cell enlargement.
Note:
- No ovarian type stroma underneath (as seen in mucinous tumours).
DDx:
- PanIN.
- Invasive ductal carcinoma of the pancreas.
- Intra-ampullary papillary-tubular neoplasm -- see ampulla of Vater.
Classification of IMPT
Commonly classified by the duct involvement:[27]
- Main duct type.
- Commonly associated with invasive carcinoma.
- Branch duct type.
- Less commonly associated with invasive carcinoma.
Behaviour - Khalifa
- Adenoma.
- Borderline mucinous tumour.
- Carcinoma.
Notes:
- Borderline tumours are rare.
- If intralobular dilated ducts... carcinoma.
- Any margin with mucin cells in thought to be badness!
Solid pseudopapillary tumour
- AKA solid pseudopapillary neoplasm, abbreviation SPN.
- AKA solid and papillary epithelial neoplasm, abbreviated SPEN.[28]
General
- Obscure cell of origin.
- Considered low grade, i.e. prognosis is usually good.
Epidemiology
Features:[29]
- Usually females (M:F=1:9).
- Mean age of presentation third decade (20s).
Management
May be followed radiologically.
Microscopic
Features:[30]
- Solid sheets of cells, focally dyscohesive.
- Eosinophilic cytoplasm.
- Occasionally clear cytoplasm.[31]
- Focal eosinophilic (intracytoplasmic) globules - key feature.
- Uniform nuclei with occasional nuclear grooves.
- +/-Necrosis - creating spaces/cavities.
- +/-Cholesterol clefts.[32]
Images
www:
DDx
- Pancreatic pseudocyst.
- Cystadenoma.
- Cystadenocarcinoma.
- Pancreatic neuroendocrine tumour - may have cytoplasmic vacuolation, hyaline globules.[31]
IHC
Features:[31]
- Beta-catenin +ve ~100% (cytoplasmic & nuclear).
- E-cadherin +ve ~100% (cytoplasmic), -ve (membrane); antibody dependent.
- CD10 +ve ~ 80% (cytoplasmic + dot-like) key.
- Synaptophysin +ve (weak cytoplasmic) ~70%.
- Progesterone receptor +ve (nuclear) key.
Others:
- CD56 +ve.
- Chromogranin -ve.
Memory device PCB: PR (nuclear), CD10 (cytoplasmic), beta-catenin (cytoplasmic & nuclear).
Pre-malignant lesions
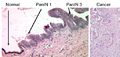
Pancreatic intraepithelial neoplasia
- Abbreviated PanIN.
General
- PanIN is thought to be the precursor lesion for pancreatic carcinoma.[33]
Overview
Putative preneoplasm-neoplasm-carcinoma sequence:
- PanIN1a.
- Not neoplastic, i.e. clonal.
- PanIN1b.
- Not neoplastic, i.e. clonal.
- PanIN2.
- Can be thought of as low-grade dysplasia, e.g. a (colonic) tubular adenoma without high-grade dysplasia.
- PanIN3.
- Can be thought of as high-grade dysplasia, e.g. (colonic) villous adenoma.
- May be referred to as carcinoma in situ.[34]
Microscopic
Features:[33]
- PanIN1a - increased amount of cytoplasm.
- Nuclear size & stratification perserved, arch. perserved.
- PanIN1b - increased amount of cytoplasm, folding of epithelium/moderated arch. distortion.
- Nuclear size & stratification perserved.
- PanIN2 - increased cell size, and nuclear enlargement (increased NC ratio), moderate nuclear atypia with loss of (basal) nuclear polarization.
- PanIN3 - marked nuclear atypia with increased NC ratio.
- No invasion identified.
- Pancreatic carcinoma - cytologic features of PanIN3 with definite invasion.
Images
www:
Solid tumours
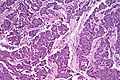
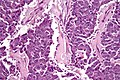
Invasive ductal carcinoma of the pancreas
General
- Most common type of pancreatic cancer.[35]
- Location: usually in the head ~60%.
- 15% in the body, 5% tail, 20% diffuse (head, body & tail).[36]
- Abysmal prognosis.
Risk factors:[37]
- Smoking (RR ~ 2).
- Pancreatitis.
- Family history, esp. BRCA2.
- Diabetes mellitus - modest risk increase.
Molecular characteristics:[38][39]
- KRAS (oncogene) mutation in ~ 90% of cases.
- CDKN2A[40] (AKA p16) inactivation ~ 95% of cases.
- TP53 (AKA p53).
- SMAD4.
Microscopic
Features:[41]
- Often glandular, may be solid.
- Nuclei.
- May be bland - little pleomorphism.
- Often small nuclei.
- Sometimes coffee-bean appearance.
- Cytoplasm - granular, abundant.
- Quasi endocrine look.
- May stain positive for endocrine markers.
Other features:
- +/-Necrosis.
- +/-Myxoid degeneration.
- +/-Cells around vessels.
DDx:
Images:
- Pancreatic adenocarcinoma (WC).
- Pancreatic adenocarcinoma (WC).
- Normal pancreas, pancreatic intraepithelial neoplasia and pancreatic carcinoma (WC).
- Pancreatic adenocarcinoma - cytopathology (WC).
- Pancreatic adenocarcinoma - several images (upmc.edu).
IHC
Features:[42]
- CD7 +ve.
- CD20 +ve.
- SMAD4 -ve ~55% of cases -- stomach usually +ve.
- CDX2 -ve/+ve.
- CEA +ve.[43]
Pancreatic neuroendocrine tumour
- Abbreviated PanNET.[44]
- AKA pancreatic islet cell tumour[44] - considered to be an outdated term.
- AKA islet cell tumour - considered to be an outdated term.
General
- Rare.
- Presentation depends on subtype, e.g. for insulinoma the typical presentation is hypoglycemia.
- May be part of a syndrome:
Classification
Based on peptide produced in the pancreatic islets:
- Glucagon from alpha cells (glucagonoma).
- Insulin from beta cells (insulinoma) - most common ~ 50% of islet cell tumours.
- Somatostatin from D cells (somatostatinoma).
- Pancreatic polypeptide from PP cells.
Others:
- Vasoactive intestinal peptide (VIPoma).
- Gastrin (gastrinoma).
- May be seen in Zollinger-Ellison syndrome.
- Triad: pancreatic gastrinoma, gastric acid hypersecretion, marked peptic ulcers in the small bowel.[47]
- May be seen in Zollinger-Ellison syndrome.
Gross
Microscopic
Features:
- Nests of cells.
- Stippled chromatin.
- +/-Hyaline globules.
DDx:
Images:
- Islet cell tumour (upmc.edu).
- Pancreatic NET with features of SPT (upmc.edu).
- Pancreatic NET - another case (upmc.edu).
IHC
- CK19 +ve -- should be done as a routine in pancreatic NETs; poor prognostic factor.[49]
Note:
- CK19 should not be confused with CA19-9.
Acinar cell carcinoma of the pancreas
- Not to be confused with acinic cell carcinoma.
General
- Rare.
- Solid epithelial exocrine tumour.[51]
- Poor prognosis; mean survival of 18 months in one series.[52]
Clinical:[52]
- Increased serum lipase.
- Associated with arthralgias (joint pain).
- Classic presentation - Schmid triad:[53]
- Subcutaneous fat necrosis.
- Polyarthritis.
- Eosinophilia.
Gross
- Usually head of pancreas.
Microscopic
Features:[52]
- Cells reminiscent of pancreatic acinus cells:
- Granular, basophilic cytoplasm - usu. abundant.
- Round/oval nucleus.
- Nucleolus prominent.
- Architecture:
- Nests, sheets, trabecular, glandular.
DDx:
Images
www:
- Acinar cell carcinoma - several images (upmc.edu).
- Acinar cell carcinoma - several images (harvard.edu).
- Acinar cell carcinoma - 1 (nature.com).
- Acinar cell carcinoma - 2 (nature.com).[54]
Stains
Features:[52]
- PAS +ve (granular).
- PASD +ve.
IHC
- Trypsin +ve -- key stain.
- Lipase +ve.
- Chromogranin +ve (scattered, focal).
- CD56 -ve. (?)
Pancreatoblastoma
General
- Very rare.
- Tumour of childhood - age of diagnosis ~5 years old.[55]
- Prognosis ~80% year survival in children[56] more aggressive in adults.
- May be seen in adults.[57]
Associations:[58]
Microscopic
- Acinar-like structures.
- Squamoid corpuscles.
- Undifferentiated component.
Image:
IHC
Features:[59]
- CK7 +ve -- acinar, undifferentiated component.
- CK8 +ve -- squamous component.
- CK18 +ve -- squamous component.
- CK19 +ve -- squamous component.
See also
- Duodenum.
- Gallbladder.
- Gastrointestinal pathology.
- Von Hippel-Lindau syndrome.
- IgG4-related systemic disease.
References
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/PancreasEndo_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SmallbowelNET_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ Jamieson, NB.; Foulis, AK.; Oien, KA.; Going, JJ.; Glen, P.; Dickson, EJ.; Imrie, CW.; McKay, CJ. et al. (Jun 2010). "Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma.". Ann Surg 251 (6): 1003-10. doi:10.1097/SLA.0b013e3181d77369. PMID 20485150.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/PancreasEndo_11protocol.pdf. Accessed on: 6 April 2012.
- ↑ URL: http://test.pathologyportal.org/newindex.htm?92nd/specgasth2.htm. Accessed on: 14 March 2011.
- ↑ 6.0 6.1 Stachura, J.; Konturek, JW.; Urbanczyk, K.; Bogdal, J.; Mach, T.; Domschke, W. (Mar 1996). "Endoscopic and histological appearance of pancreatic metaplasia in the human gastric mucosa: a preliminary report on a recently recognized new type of gastric mucosal metaplasia.". Eur J Gastroenterol Hepatol 8 (3): 239-43. PMID 8724024.
- ↑ Johansson J, Håkansson HO, Mellblom L, et al. (March 2010). "Pancreatic acinar metaplasia in the distal oesophagus and the gastric cardia: prevalence, predictors and relation to GORD". J. Gastroenterol. 45 (3): 291–9. doi:10.1007/s00535-009-0161-4. PMID 20012917.
- ↑ Doglioni, C.; Laurino, L.; Dei Tos, AP.; De Boni, M.; Franzin, G.; Braidotti, P.; Viale, G. (Nov 1993). "Pancreatic (acinar) metaplasia of the gastric mucosa. Histology, ultrastructure, immunocytochemistry, and clinicopathologic correlations of 101 cases.". Am J Surg Pathol 17 (11): 1134-43. PMID 8214258.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 223. ISBN 978-1416002741.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 469. ISBN 978-1416054542.
- ↑ URL: http://path.upmc.edu/cases/case651/dx.html. Accessed on: 28 January 2012.
- ↑ 12.0 12.1 Kamisawa, T.; Takuma, K.; Tabata, T.; Inaba, Y.; Egawa, N.; Tsuruta, K.; Hishima, T.; Sasaki, T. et al. (Jan 2011). "Serum IgG4-negative autoimmune pancreatitis.". J Gastroenterol 46 (1): 108-16. doi:10.1007/s00535-010-0317-2. PMID 20824290.
- ↑ Ikeura, T.; Takaoka, M.; Uchida, K.; Shimatani, M.; Miyoshi, H.; Kusuda, T.; Kurishima, A.; Fukui, Y. et al. (2012). "Autoimmune pancreatitis with histologically proven lymphoplasmacytic sclerosing pancreatitis with granulocytic epithelial lesions.". Intern Med 51 (7): 733-7. PMID 22466829.
- ↑ Krasinskas, AM.; Raina, A.; Khalid, A.; Tublin, M.; Yadav, D. (Jun 2007). "Autoimmune pancreatitis.". Gastroenterol Clin North Am 36 (2): 239-57, vii. doi:10.1016/j.gtc.2007.03.015. PMID 17533077.
- ↑ 15.0 15.1 15.2 Adsay, NV.; Bandyopadhyay, S.; Basturk, O.; Othman, M.; Cheng, JD.; Klöppel, G.; Klimstra, DS. (Nov 2004). "Chronic pancreatitis or pancreatic ductal adenocarcinoma?". Semin Diagn Pathol 21 (4): 268-76. PMID 16273946.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 225. ISBN 978-1416002741.
- ↑ Hornick, JL.; Lauwers, GY.; Odze, RD. (Mar 2005). "Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver.". Am J Surg Pathol 29 (3): 381-9. PMID 15725808.
- ↑ URL: http://www.joplink.net/prev/200905/25.html. Accessed on: 15 February 2012.
- ↑ 19.0 19.1 URL: http://oac.med.jhmi.edu/cpc/cases/cpc5/cpc5_answer.html. Accessed on: 15 February 2012.
- ↑ 20.0 20.1 Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Oberman, Harold A; Reuter, Victor E (2004). Sternberg's Diagnostic Surgical Pathology (4th ed.). Lippincott Williams & Wilkins. pp. 1630. ISBN 978-0781740517.
- ↑ Bano, S.; Upreti, L.; Puri, SK.; Chaudhary, V.; Sakuja, P. (Dec 2011). "Imaging of pancreatic serous cystadenocarcinoma.". Jpn J Radiol 29 (10): 730-4. doi:10.1007/s11604-011-0617-3. PMID 22009426.
- ↑ Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH (2005). "Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst". Radiographics 25 (3): 671–85. doi:10.1148/rg.253045104. PMID 15888617. http://radiographics.rsna.org/content/25/3/671.abstract.
- ↑ Vernadakis, S.; Kaiser, GM.; Christodoulou, E.; Mathe, Z.; Troullinakis, M.; Bankfalvi, A.; Paul, A. (2009). "Enormous serous microcystic adenoma of the pancreas.". JOP 10 (3): 332-4. PMID 19454830.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 489. ISBN 978-0443066573.
- ↑ Maire F, Hammel P, Terris B, et al. (November 2002). "Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma". Gut 51 (5): 717–22. PMC 1773420. PMID 12377813. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=12377813.
- ↑ Baiocchi GL, Portolani N, Missale G, et al. (2010). "Intraductal papillary mucinous neoplasm of the pancreas (IPMN): clinico-pathological correlations and surgical indications". World J Surg Oncol 8: 25. doi:10.1186/1477-7819-8-25. PMC 2858722. PMID 20374620. http://wjso.com/content/8/1/25.
- ↑ Ikeuchi, N.; Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S. et al. (Apr 2010). "Prognosis of cancer with branch duct type IPMN of the pancreas.". World J Gastroenterol 16 (15): 1890-5. PMID PMC = 2856831 20397268 PMC = 2856831.
- ↑ URL: http://brighamrad.harvard.edu/Cases/bwh/hcache/360/full.html. Accessed on: 31 October 2011.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 493. ISBN 978-0443066573.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 493-5. ISBN 978-0443066573.
- ↑ 31.0 31.1 31.2 Serra S, Chetty R (November 2008). "Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas". J. Clin. Pathol. 61 (11): 1153–9. doi:10.1136/jcp.2008.057828. PMID 18708424. http://jcp.bmj.com/content/61/11/1153.
- ↑ Abad Licham, M.; Sanchez Lihon, J.; Celis Zapata, J.. "[Pseudopapillary solid tumor of pancreas in the INEN].". Rev Gastroenterol Peru 28 (4): 356-61. PMID 19156179.
- ↑ 33.0 33.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 949. ISBN 0-7216-0187-1.
- ↑ Matthaei, H.; Hong, SM.; Mayo, SC.; dal Molin, M.; Olino, K.; Venkat, R.; Goggins, M.; Herman, JM. et al. (Nov 2011). "Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer.". Ann Surg Oncol 18 (12): 3493-9. doi:10.1245/s10434-011-1745-9. PMID 21537863.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 237. ISBN 978-0781765275.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 950. ISBN 0-7216-0187-1.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 471. ISBN 978-1416054542.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 470-1. ISBN 978-1416054542.
- ↑ Furukawa, T. (Nov 2009). "Molecular pathology of pancreatic cancer: implications for molecular targeting therapy.". Clin Gastroenterol Hepatol 7 (11 Suppl): S35-9. doi:10.1016/j.cgh.2009.07.035. PMID 19896096.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600160
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 951. ISBN 0-7216-0187-1.
- ↑ Lester, Susan Carole (2010). Manual of Surgical Pathology (3rd ed.). Saunders. pp. 94. ISBN 978-0-323-06516-0.
- ↑ Adsay, NV.; Basturk, O.; Cheng, JD.; Andea, AA. (Oct 2005). "Ductal neoplasia of the pancreas: nosologic, clinicopathologic, and biologic aspects.". Semin Radiat Oncol 15 (4): 254-64. doi:10.1016/j.semradonc.2005.04.001. PMID 16183479.
- ↑ 44.0 44.1 Burns, WR.; Edil, BH. (Dec 2011). "Neuroendocrine Pancreatic Tumors: Guidelines for Management and Update.". Curr Treat Options Oncol. doi:10.1007/s11864-011-0172-2. PMID 22198808.
- ↑ Charlesworth, M.; Verbeke, CS.; Falk, GA.; Walsh, M.; Smith, AM.; Morris-Stiff, G. (Feb 2012). "Pancreatic Lesions in von Hippel-Lindau Disease? A Systematic Review and Meta-synthesis of the Literature.". J Gastrointest Surg. doi:10.1007/s11605-012-1847-0. PMID 22370733.
- ↑ Alexakis, N.; Connor, S.; Ghaneh, P.; Lombard, M.; Smart, HL.; Evans, J.; Hughes, M.; Garvey, CJ. et al. (2004). "Hereditary pancreatic endocrine tumours.". Pancreatology 4 (5): 417-33; discussion 434-5. doi:10.1159/000079616. PMID 15249710.
- ↑ Zollinger RM, Ellison EH (1955). "Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas". Ann. Surg. 142 (4): 709–23; discussion, 724–8. doi:10.1097/00000658-195510000-00015. PMC 1465210. PMID 13259432. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1465210/.
- ↑ 48.0 48.1 Oh, TG.; Chung, MJ.; Park, JY.; Bang, SM.; Park, SW.; Chung, JB.; Song, SY. (Sep 2012). "Prognostic factors and characteristics of pancreatic neuroendocrine tumors: single center experience.". Yonsei Med J 53 (5): 944-51. doi:10.3349/ymj.2012.53.5.944. PMC 3423842. PMID 22869477. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3423842/. Cite error: Invalid
<ref>tag; name "pmid22869477" defined multiple times with different content - ↑ Jain, R.; Fischer, S.; Serra, S.; Chetty, R. (Jan 2010). "The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver.". Appl Immunohistochem Mol Morphol 18 (1): 9-15. doi:10.1097/PAI.0b013e3181ad36ea. PMID 19956064.
- ↑ Thomas, PC.; Nash, GF.; Aldridge, MC. (2003). "Pancreatic acinar cell carcinoma presenting as acute pancreatitis.". HPB (Oxford) 5 (2): 111-3. doi:10.1080/13651820310001153. PMID 18332967.
- ↑ URL: http://brighamrad.harvard.edu/Cases/bwh/hcache/380/full.html. Accessed on: 15 January 2012.
- ↑ 52.0 52.1 52.2 52.3 Klimstra, DS.; Heffess, CS.; Oertel, JE.; Rosai, J. (Sep 1992). "Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases.". Am J Surg Pathol 16 (9): 815-37. PMID 1384374.
- ↑ Jang, SH.; Choi, SY.; Min, JH.; Kim, TW.; Lee, JA.; Byun, SJ.; Lee, JW. (Feb 2010). "[A case of acinar cell carcinoma of pancreas, manifested by subcutaneous nodule as initial clinical symptom].". Korean J Gastroenterol 55 (2): 139-43. PMID 20168061.
- ↑ 54.0 54.1 Klimstra, DS. (Feb 2007). "Nonductal neoplasms of the pancreas.". Mod Pathol 20 Suppl 1: S94-112. doi:10.1038/modpathol.3800686. PMID 17486055.
- ↑ Glick, RD.; Pashankar, FD.; Pappo, A.; Laquaglia, MP. (May 2012). "Management of pancreatoblastoma in children and young adults.". J Pediatr Hematol Oncol 34 Suppl 2: S47-50. doi:10.1097/MPH.0b013e31824e3839. PMID 22525406.
- ↑ Bien, E.; Godzinski, J.; Dall'igna, P.; Defachelles, AS.; Stachowicz-Stencel, T.; Orbach, D.; Bisogno, G.; Cecchetto, G. et al. (Oct 2011). "Pancreatoblastoma: a report from the European cooperative study group for paediatric rare tumours (EXPeRT).". Eur J Cancer 47 (15): 2347-52. doi:10.1016/j.ejca.2011.05.022. PMID 21696948.
- ↑ Balasundaram, C.; Luthra, M.; Chavalidthamrong, D.; Chow, J.; Khan, H.; Endres, PJ. (May 2012). "Pancreatoblastoma: a rare tumor still evolving in clinical presentation and histology.". JOP 13 (3): 301-3. PMID 22572137.
- ↑ 58.0 58.1 Saif, MW. (2007). "Pancreatoblastoma.". JOP 8 (1): 55-63. PMID 17228135.
- ↑ 59.0 59.1 Nishimata, S.; Kato, K.; Tanaka, M.; Ijiri, R.; Toyoda, Y.; Kigasawa, H.; Ohama, Y.; Nakatani, Y. et al. (Jun 2005). "Expression pattern of keratin subclasses in pancreatoblastoma with special emphasis on squamoid corpuscles.". Pathol Int 55 (6): 297-302. doi:10.1111/j.1440-1827.2005.01829.x. PMID 15943785.
Further reading
Klimstra, DS.; Pitman, MB.; Hruban, RH. (Mar 2009). "An algorithmic approach to the diagnosis of pancreatic neoplasms.". Arch Pathol Lab Med 133 (3): 454-64. doi:10.1043/1543-2165-133.3.454. PMID 19260750.