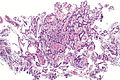
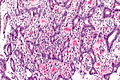
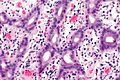
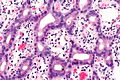
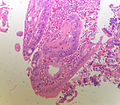
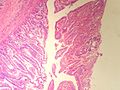
Ampulla of Vater
The ampulla of Vater, also hepatopancreatic ampulla, is found in the duodenum. It has a unique histology and is a relatively common site of disease, when duodenal pathology is considered.
Benign
Normal histology
Periampullary:[1]
- Intestinal epithelium with goblet cells.
Papilla of Vater (the projection into the duodenal lumen):[1]
- Goblet cells in foveolar-like epithelium.
Note about heterotopias:[2]
- +/-Pancreatic heterotopia - common.
- +/-Gastric heterotopia - not common.
Sign out
DUODENAL BULB, BIOPSY: - GASTRIC HETEROTOPIA. - NEGATIVE FOR MALIGNANCY.
Acute inflammation
Sign out
Ampulla of Vater, Biopsy: - Small bowel mucosa with foveolar-like glands (in keeping with ampulla), reactive epithelial changes and mild acute inflammation. - NEGATIVE for dysplasia.
Ampullary tumours
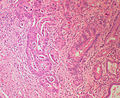
Ampullary adenoma
General
- Uncommon.
- May be associated with familial adenomatous polyposis (FAP).
Microscopic
Features:
- +/-Paneth cells - may be prominent.[3]
- Similar to adenoma of colon - with:
- Less pseudostratification.
- Finer chromatin pattern.
DDx:
- Ampullary carcinoma.
- Duodenal carcinoma secondarily involving the ampulla.
- Pancreatic adenocarcinoma secondarily involving the ampulla.
Images
www:
Sign out
- See tubular adenoma.
Ampullary carcinoma
- AKA ampullary adenocarcinoma.
General
- Uncommon.
- Textbook association: familial adenomatous polyposis.[5][6]
- Prognosis guarded but significantly better than pancreatic ductal adenocarcinoma - 5 year survival ~40% for ampullary carcinomas vs. 10% for pancreatic adenocarcinoma.[1]
Gross
- Ampullary carcinomas are classified by site.
- Modest differences exist in survival between the sites.
Classification
Adsay et al. proposed a four subtype classification system:[1]
| Subtype | Prevalence | Origin/definition | Notes |
|---|---|---|---|
| Intra-ampullary papillary-tubular carcinoma | ~25% of cases | arises from intra-ampullary epithelium and/or distal end of CBD or pancreatic duct | generalized category intra-ampullary papillary-tubular neoplasm -- the ampullary counterpart of the IPMN of the pancreas |
| Ampullary-ductal carcinoma | ~15% of cases | arises from intra-ampullary ducts | |
| Peri-ampullary duodenal carcinoma | ~5% of cases | primarily in the duodenum; ampullary orfice must be clearly within lesion | |
| Ampullary carcinoma not otherwise specified | ~55% of cases | arise from papillary projection into duodenum - from foveolar-like epithelium with goblet cells |
Microscopic
Dependent on histologic subtype:[1][7]
- Intestinal ampullary carcinoma.
- Pancreaticobiliary ampullary carcinoma.
- Other.
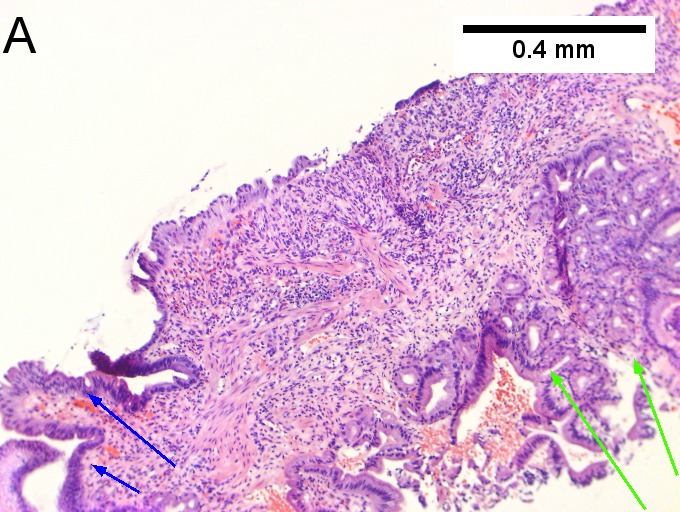
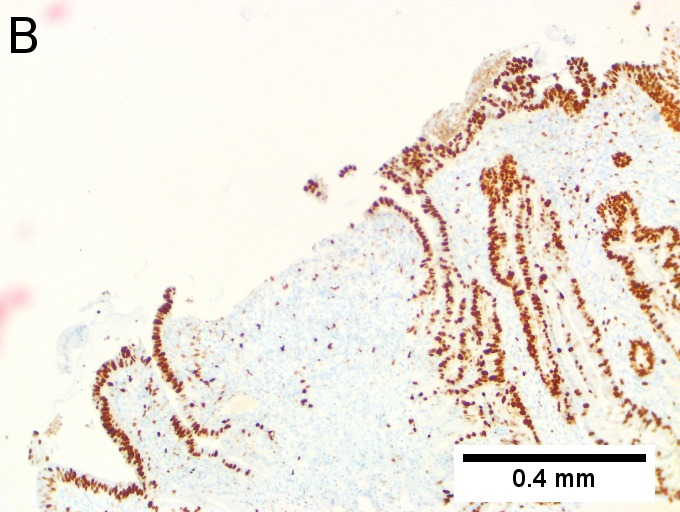
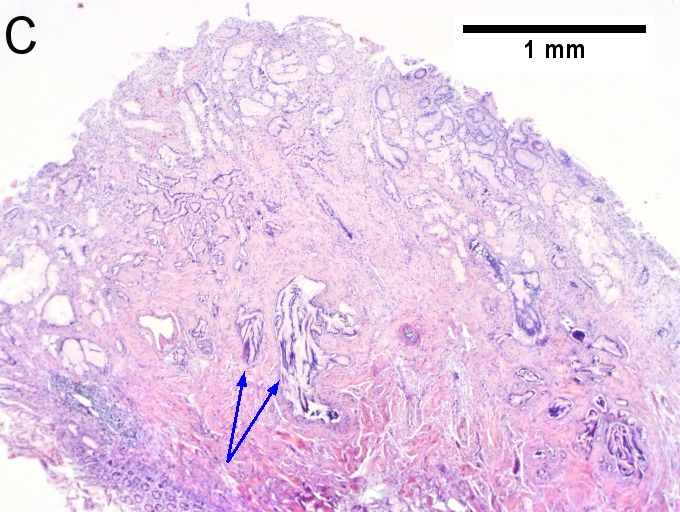
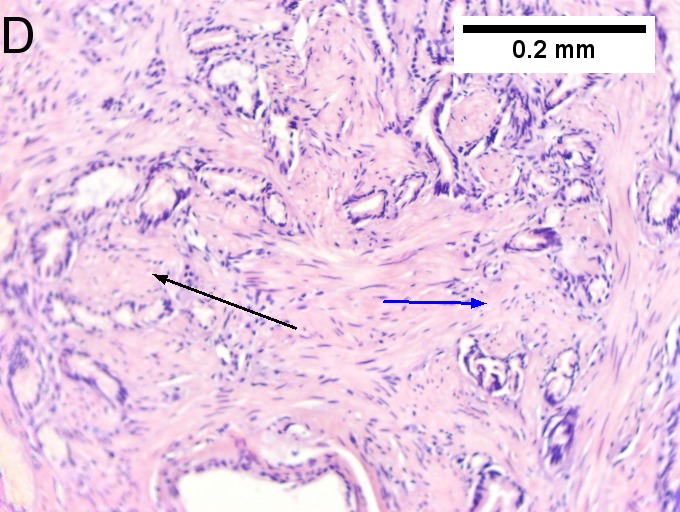
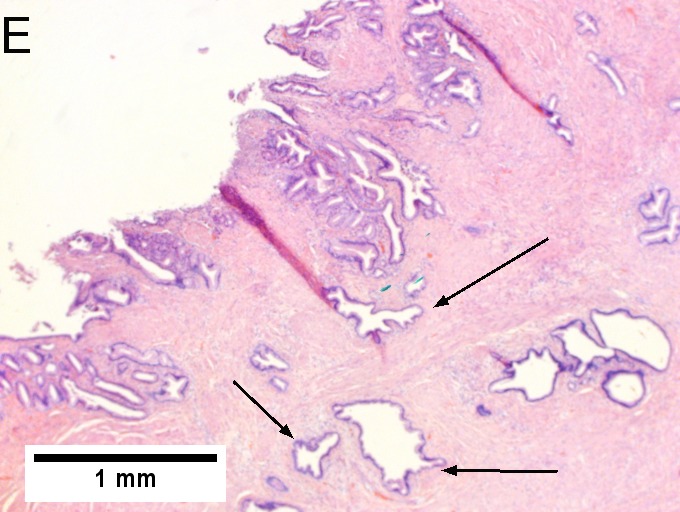
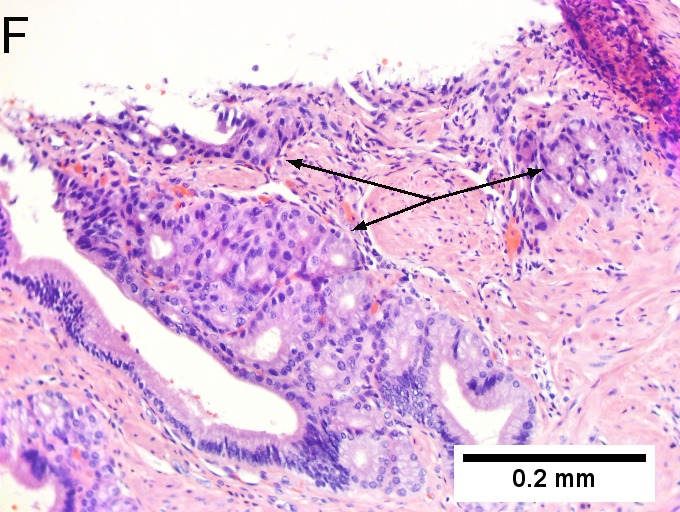
Invasive carcinoma of duodenal ampulla, intestinal type. A. Ulceration tops Brunner’s glands [green arrow]; at the edge lie glands [blue arrow] with changes suggesting adenoma (100X). B. Ki67 stain establishes adenoma by surface extension of brown nuclei (100X). C. The lesion was a mass, prompting rebiopsy. The cauterized fragment shows disorderly spread of glands, with dilated glands at base [arrows] not readily explained by obstruction (40X) D.Two cancerous prongs, one on left [black arrow], one on right [blue arrow] each show spread about muscle fibers (200X). E. The Whipple resection showed the same dilated spreading glands [arrows] at base, redolent of the spread of some well-differentiated colonic adenocarcinomas through muscle (40X). F. Cribriformed cancerous nests [arrows] cannot be Brunner’s glands, because the nuclei are too variable and lack polarity and because they abut crypts extending to the surface; cautery would have made this an impossible distinction (200X).
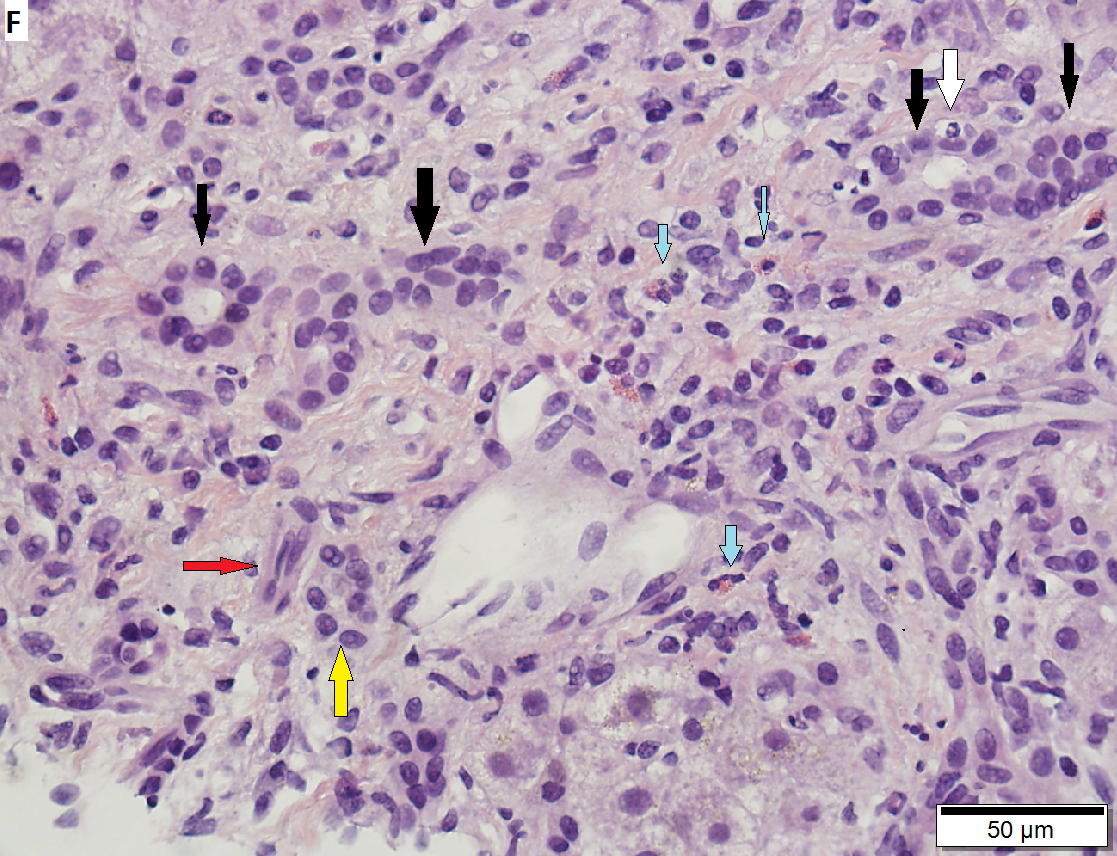
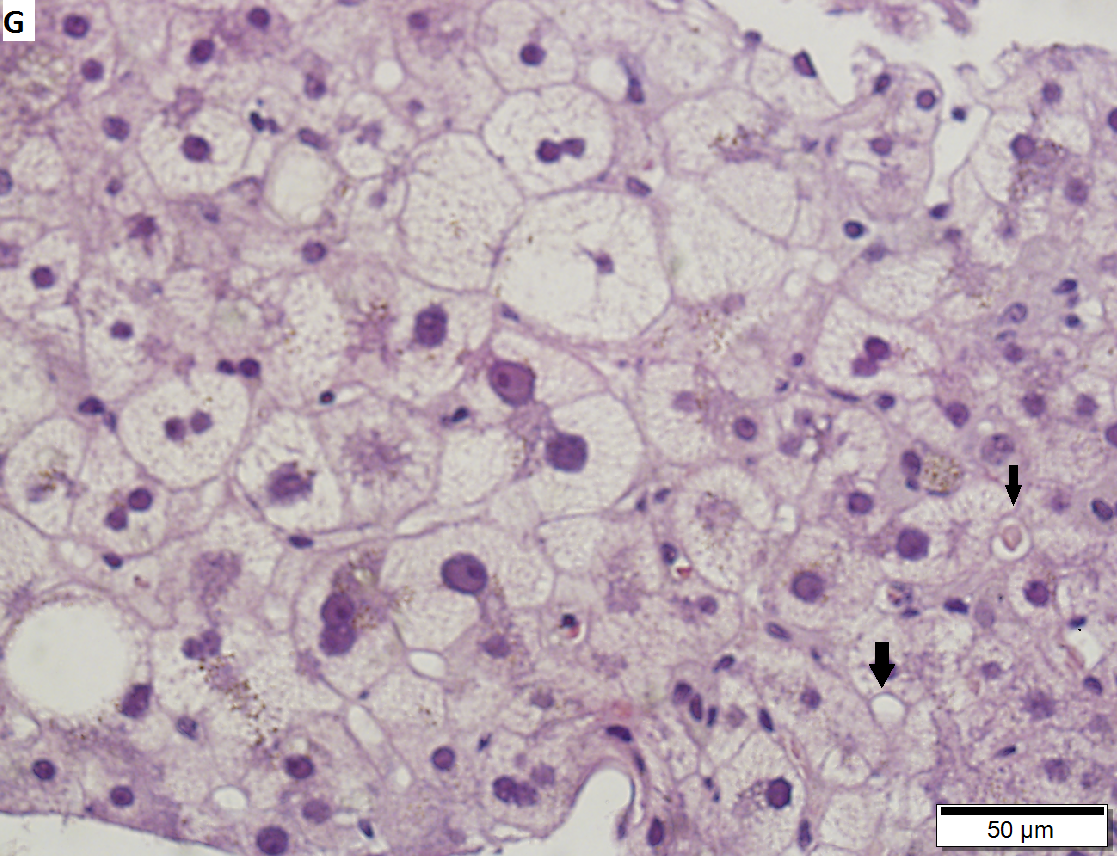
60 year old woman with poorly differentiated ampullary adenocarcinoma and obstructive cholestasis in liver. A. An isolated tumor fragment (black arrow) and two fragments with tumor undermining small intesatinal mucosa (green arrows) are seen. B. Large, ovoid cancer nuclei with abundant grey cytoplasm form sheets with only rare acini (arrow). C. Tumor cells are CK7 positive. D. Tumor cells are CK20 negative. E. Portal triads (black arrows) are expanded, lacking intense inflammation. A septal duct (green arrow) lacks definite onion skinning. F. Proliferated peripheral bile ductules (black arrows) sometimes have neutrophils (white arrow), which should not be interpreted as ascending cholangitis. Next to the artery (red arrow) lies the bile duct (yellow arrow), which has disturbed nuclei, but no neutrophils. Note occasional eosinophils (cyan arrows). G. Hepatocytes with feathery degeneration. Arrows point to dilated cholangioles, one filled with a bile plug.
Notes:
- May lack desmoplastic stroma.[3]
DDx:
- Invasive ductal carcinoma of the pancreas - has a much worse prognosis.
- Ectopic pancreas.[8]
- Duodenal adenocarcinoma with secondary involvement of the ampulla.
Intestinal ampullary carcinoma
Features:[1]
- Pseudostratified columnar epithelium with hyperchromatic, ellipsoid nuclei.
- Glands usually tightly packed, i.e. high gland-to-stroma ratio; often >2:1.
Note:
- Similar to colorectal adenocarcinoma.
Pancreatobiliary ampullary carcinoma
Features:[1]
- Tubular arrangements consisting of cuboidal cells in one or two layers.
- Tubules usually spaced; ~1:3 gland-to-stroma ratio.
Images
Other
Features - any of the following characteristics:[1]
- Non-tubular morphology/poorly-differentiated.
- Micropapillary architecture.
- Medullary.
- Signet ring cells.
- Mucin:
- Colloid.
- Mixed-mucinous.
- Mucinous-signet-ring.
IHC
Features:[7]
- CK7 +ve.
- CK20 +ve.
- MUC2 +ve.
Others:
- SMAD4 +ve/-ve.
- Lost in pancreatic neoplasia ~90% of cases vs. ~35% of ampullary tumours.[9]
- p53 +ve ~55% of cases.[10][11]
- E-cadherin +ve ~40% of cases.[12]
- Beta-catenin +ve ~65% of cases.[12]
Sign out
- Separate CAP protocol.[13]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Adsay, V.; Ohike, N.; Tajiri, T.; Kim, GE.; Krasinskas, A.; Balci, S.; Bagci, P.; Basturk, O. et al. (Sep 2012). "Ampullary Region Carcinomas: Definition and Site Specific Classification with Delineation of Four Clinicopathologically and Prognostically Distinct Subsets in an Analysis of 249 Cases.". Am J Surg Pathol. doi:10.1097/PAS.0b013e31826399d8. PMID 23026934.
- ↑ Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Reuter, Victor E; Stoler, Mark H (2009). Sternberg's Diagnostic Surgical Pathology (5th ed.). Lippincott Williams & Wilkins. pp. 1639. ISBN 978-0781779425.
- ↑ 3.0 3.1 Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Reuter, Victor E; Stoler, Mark H (2009). Sternberg's Diagnostic Surgical Pathology (5th ed.). Lippincott Williams & Wilkins. pp. 1640. ISBN 978-0781779425.
- ↑ 4.0 4.1 Bellizzi, AM.; Kahaleh, M.; Stelow, EB. (Oct 2009). "The assessment of specimens procured by endoscopic ampullectomy.". Am J Clin Pathol 132 (4): 506-13. doi:10.1309/AJCPUZWJ8WA2IHBG. PMID 19762527.
- ↑ Tran, TC.; Vitale, GC. (Dec 2004). "Ampullary tumors: endoscopic versus operative management.". Surg Innov 11 (4): 255-63. PMID 15756395.
- ↑ Soravia, C.; Berk, T.; Haber, G.; Cohen, Z.; Gallinger, S.. "Management of advanced duodenal polyposis in familial adenomatous polyposis.". J Gastrointest Surg 1 (5): 474-8. PMID 9834381.
- ↑ 7.0 7.1 Fischer, HP.; Zhou, H. (2004). "Pathogenesis of carcinoma of the papilla of Vater.". J Hepatobiliary Pancreat Surg 11 (5): 301-9. doi:10.1007/s00534-004-0898-3. PMID 15549428.
- ↑ Hsu, SD.; Chan, DC.; Hsieh, HF.; Chen, TW.; Yu, JC.; Chou, SJ. (Apr 2008). "Ectopic pancreas presenting as ampulla of Vater tumor.". Am J Surg 195 (4): 498-500. doi:10.1016/j.amjsurg.2007.01.043. PMID 18304504.
- ↑ McCarthy, DM.; Hruban, RH.; Argani, P.; Howe, JR.; Conlon, KC.; Brennan, MF.; Zahurak, M.; Wilentz, RE. et al. (Mar 2003). "Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of Vater: analysis of 140 cases.". Mod Pathol 16 (3): 272-8. doi:10.1097/01.MP.0000057246.03448.26. PMID 12640108.
- ↑ Takashima, M.; Ueki, T.; Nagai, E.; Yao, T.; Yamaguchi, K.; Tanaka, M.; Tsuneyoshi, M. (Dec 2000). "Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions.". Mod Pathol 13 (12): 1300-7. doi:10.1038/modpathol.3880238. PMID 11144926.
- ↑ Park, SH.; Kim, YI.; Park, YH.; Kim, SW.; Kim, KW.; Kim, YT.; Kim, WH. (Jan 2000). "Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of Vater.". World J Surg 24 (1): 54-9. PMID 10594204.
- ↑ 12.0 12.1 Park, S.; Kim, SW.; Lee, BL.; Jung, EJ.; Kim, WH.. "Expression of E-cadherin and beta-catenin in the adenoma-carcinoma sequence of ampulla of Vater cancer.". Hepatogastroenterology 53 (67): 28-32. PMID 16506371.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Ampulla_12protocol_3101.pdf. Accessed on: 12 September 2012.