Difference between revisions of "Liver metastasis"
(→Melanoma: added case) |
|||
| (3 intermediate revisions by one other user not shown) | |||
| Line 9: | Line 9: | ||
| LMDDx = primary liver lesions ([[cholangiocarcinoma]], [[hepatocellular carcinoma]] (esp. pseudoglandular), [[epithelioid hemangioendothelioma]]) | | LMDDx = primary liver lesions ([[cholangiocarcinoma]], [[hepatocellular carcinoma]] (esp. pseudoglandular), [[epithelioid hemangioendothelioma]]) | ||
| Stains = | | Stains = | ||
| IHC = HepPar-1 -ve, CK20 +ve (colorectal carcinoma) | | IHC = [[HepPar-1]] -ve, [[CK20]] +ve (colorectal carcinoma) | ||
| EM = | | EM = | ||
| Molecular = | | Molecular = | ||
| Line 79: | Line 79: | ||
==IHC== | ==IHC== | ||
*Metastases are typically negative for ''HepPar-1''. | *Metastases are typically negative for ''HepPar-1''. | ||
**HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | **[[HepPar-1]] (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | ||
A panel: | A panel: | ||
| Line 113: | Line 113: | ||
====Micro==== | ====Micro==== | ||
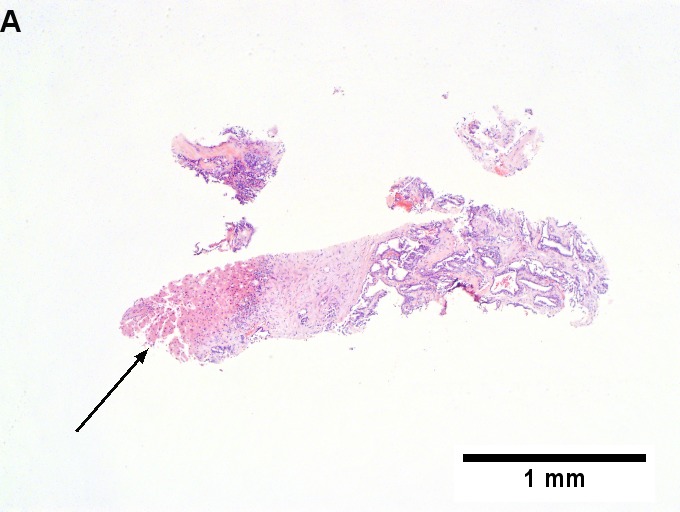
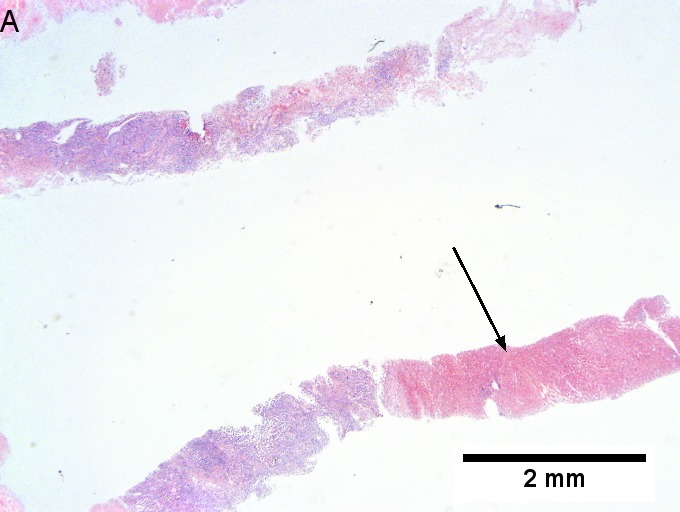
The section show liver parenchyma with an invasive adenocarcinoma. The adenocarcinoma | |||
has well formed glands with dirty necrosis. The nuclei are appear crowded and | |||
have an ellipsoid shape. Focally, zones of necrosis are present. See background liver. | |||
BACKGROUND LIVER (BASED ON H&E ONLY)<br> | |||
Fibrosis: not identified.<br> | |||
Fibrous septa: absent.<br> | |||
Septa with curved contours: absent.<br> | |||
Large droplet steatosis (% of hepatocytes): mild (20%).<br> | |||
Ballooning of hepatocytes: not identified.<br> | |||
Mallory-Denk bodies: not identified.<br> | |||
Portal inflammation: present, mild.<br> | |||
Interface activity: not identified.<br> | |||
Lobular necroinflammation: not identified.<br> | |||
Ducts: present in normal numbers.<br> | |||
Duct injury: not identified.<br> | |||
Ductular reaction: not identified.<br> | |||
Cholestasis: present peritumoural, otherwise absent.<br> | |||
Terminal hepatic venules: present.<br> | |||
Ground glass cells with routine stains: not identified. | |||
[[File: COL MET 1 sl 1.png| Metastasis of colonic carcinoma to liver]] | |||
[[File: COL MET 1 sl 2.png| Metastasis of colonic carcinoma to liver]] | |||
[[File: COL MET 1 sl 3.png| Metastasis of colonic carcinoma to liver]] | |||
[[File: COL MET 1 sl 4.png| Metastasis of colonic carcinoma to liver]] | |||
[[File: COL MET 1 sl 5.png| Metastasis of colonic carcinoma to liver]] | |||
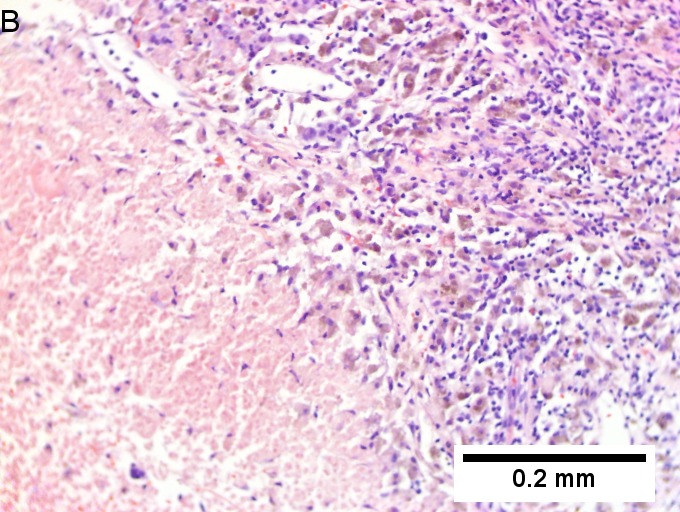
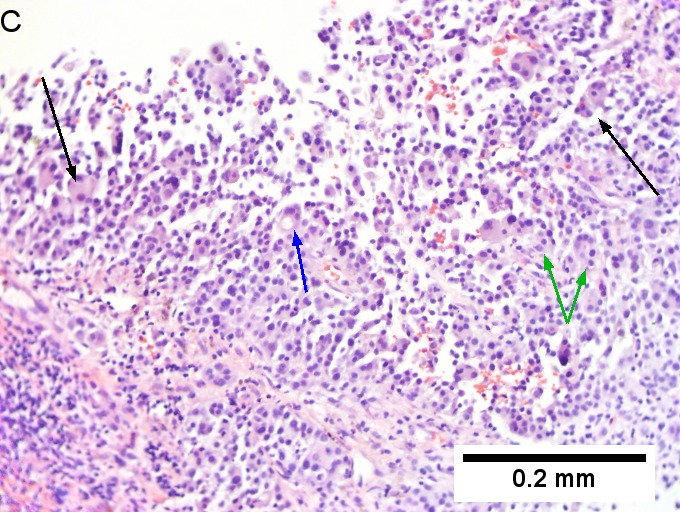
Metastasis of colonic carcinoma to liver. A. Blue arrow shows colon cancer with large acini. Green arrow shows necrotic tumor. Black arrow shows sinusoidal dilatation, an example of a nonspecific tumor associated change that should not be deemed systemic in nature. B. Elongated nuclei perpendicular to acinar border (green arrows) and dirty luminal necrosis (black arrow) are characteristic of colon cancer. C. Immunostain for CK7. Positive groups may be focal staining by tumor, but more likely are residual bile ductules. D. Immunostain for CK20 is more diffusely positive and definitely in tumor. | |||
[[File: SQC MET 1 sl 1.png| Metastasis of squamous carcinoma to liver]] | |||
[[File: SQC MET 1 sl 2.png| Metastasis of squamous carcinoma to liver]] | |||
[[File: SQC MET 1 sl 3.png| Metastasis of squamous carcinoma to liver]] | |||
[[File: SQC MET 1 sl 4.png| Metastasis of squamous carcinoma to liver]] | |||
[[File: SQC MET 1 sl 5.png| Metastasis of squamous carcinoma to liver]] | |||
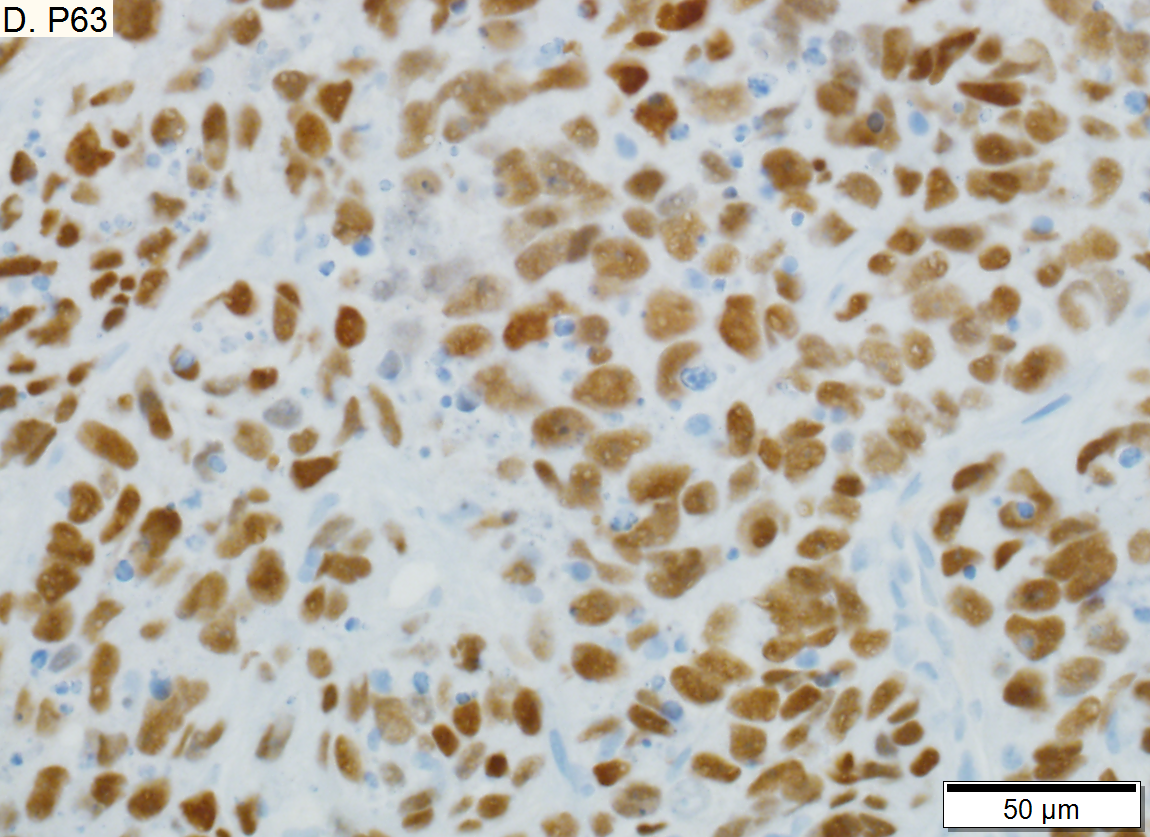
Metastasis of Squamous carcinoma to liver. A. Dark blue tumor groups mass to replace liver.B. Carcinomatous nests show scant stromal separation, with individual cancer cells being a the edge of the necrotic space C. Huge nucle show nucleoli with occasional spindleds cell nuclei. Individual cell necrosis is prominent. Cytoplasm is scant. Immunostain for CK7. Positive groups may be focal staining by tumor, but more likely are residual bile ductules. D. Immunostain for P63 is positive; CK7, CK20, chromogranin, synaptophysin, and TTF1 were negative | |||
{| | {| | ||
[[File:1 Met scc 1 680x512px.tif|Metastatic poorly differentiated neuroendocrine carcinoma of pulmonary origin.]] | [[File:1 Met scc 1 680x512px.tif|Metastatic poorly differentiated neuroendocrine carcinoma of pulmonary origin.]] | ||
| Line 134: | Line 173: | ||
Metastatic thyroid carcinoma. A. Carcinoma with open spaces spares part of a normal liver lobule [arrow] (40X). B. A sheet with acini [arrows] would indicate only adenocarcinoma (400X). C. A true papilla with vascular spaces [arrows] suggests papillary carcinoma (400X). D. Some spaces show tumor nuclei with inclusions [arrows] (400X). E. Tumor nuclei are TTF1 positive, which can also be seen with lung cancer (IHC 400X). F. Specific thyroglobulin positivity rendered the specific diagnosis known (IHC 400X). | Metastatic thyroid carcinoma. A. Carcinoma with open spaces spares part of a normal liver lobule [arrow] (40X). B. A sheet with acini [arrows] would indicate only adenocarcinoma (400X). C. A true papilla with vascular spaces [arrows] suggests papillary carcinoma (400X). D. Some spaces show tumor nuclei with inclusions [arrows] (400X). E. Tumor nuclei are TTF1 positive, which can also be seen with lung cancer (IHC 400X). F. Specific thyroglobulin positivity rendered the specific diagnosis known (IHC 400X). | ||
[[File: RCC MET 1 sl 1.png| Metastasis of renal cell carcinoma to liver]] | |||
[[File: RCC MET 1 sl 2.png| Metastasis of renal cell carcinoma to liver]] | |||
[[File: RCC MET 1 sl 3.png| Metastasis of renal cell carcinoma to liver]] | |||
[[File: RCC MET 1 sl 4.png| Metastasis of renal cell carcinoma to liver]] | |||
[[File: RCC MET 1 sl 5.png| Metastasis of renal cell carcinoma to liver]] | |||
Metastasis of renal cell carcinoma to liver. A. Upper core shows blue necrosis; remainder shows replacement by tumor. B. In part cytoplasm is abundant and amphophilic, suggesting hepatocellular carcinoma. C. In part, tumor shows clear cytoplasm, raising alternative explanations. D. High power of clear area shows finely reticulated cytoplasm with sharp intercellular borders. Nuclei are bizarrely shaped, often with nucleoli. Arrow shows an aberrant mitosis. E. Immunostain for RCC proves renal cell carcinoma. | |||
===Melanoma=== | ===Melanoma=== | ||
Latest revision as of 16:21, 22 November 2019
| Liver metastasis | |
|---|---|
| Diagnosis in short | |
 Metastatic adenocarcinoma. H&E stain. | |
|
| |
| Synonyms | hepatic metastasis |
|
| |
| LM | lack of identifiable bile, usually not cirrhotic, variable morphology - commonly adenocarcinoma |
| Subtypes | commonly colorectal carcinoma, gastric adenocarcinoma, pancreatic adenocarcinoma |
| LM DDx | primary liver lesions (cholangiocarcinoma, hepatocellular carcinoma (esp. pseudoglandular), epithelioid hemangioendothelioma) |
| IHC | HepPar-1 -ve, CK20 +ve (colorectal carcinoma) |
| Site | liver - see liver neoplasms |
|
| |
| Clinical history | +/-history of colorectal carcinoma |
| Radiology | hypoattenuating nodule on CT, hypodense lesions in venous phase on CT with contrast |
| Prognosis | dependent on stage and primary site, usually poor |
| Clin. DDx | hepatic abscess |
| Treatment | liver resection if isolated and feasible |
A liver metastasis is a malignant metastatic neoplasm in the liver that arose elsewhere. Liver metastases are more common than primary liver neoplasms.
It is also known as metastatic liver disease and hepatic metastasis.
Metastatic disease in general is dealt with in the metastasis article.
General
- Metastases are very common - often from the gastrointestinal tract, e.g. colorectal cancer.
- Most liver masses in are not biopsied... as a primary lesion is evident.[1]
- Dependent on the extent of disease, CRC metastatic to the liver may be curable with a liver resection.
- Peritoneal disease, i.e. a malignant peritoneal nodule, in the context of liver metastases does poorly, and is considered a contraindication to liver resection.[2]
- It is important to consider germ cell tumours in the DDx as these may be curable with chemotherapy.
- Clear cell variant of HCC may be misdiagnosed as metastatic clear cell carcinoma.
- Interhepatic cholangiocarcinoma is an adenocarcinoma - it may look like a metastatic lesion.
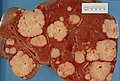
Gross pathology/radiology
Features:
- Multifocal or solitary.
- Classically multifocal.
Radiology:
- Classically hypoattenuating nodule on CT - may be confused with abscess radiologically.[3]
- Hypodense lesions in venous phase on CT with contrast.[4][5]
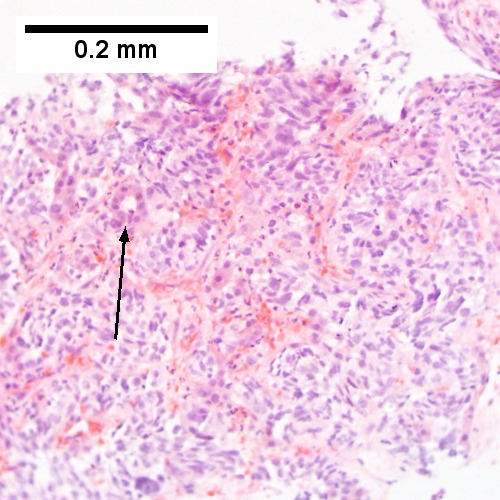
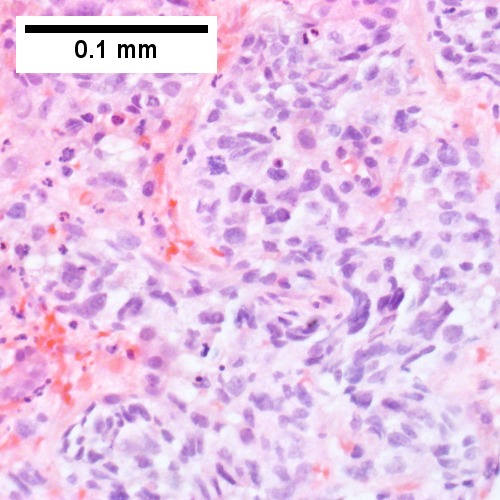
Images
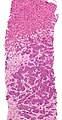
Microscopic
Features:
- Histologic features are dependent on primary and degree of differentiation.
The classic liver metastasis (colorectal carcinoma):
- Gland forming columnar shaped cells with pseudostratified hyperchromatic cigar-shaped nuclei.
DDx:
- Cholangiocarcinoma.
- Hepatocellular carcinoma, pseudoglandular.[6]
- Epithelioid hemangioendothelioma. (???)
Image
IHC
- Metastases are typically negative for HepPar-1.
A panel:
- CK7, CK20, TTF-1, CDX2, CA-125.[8]
Note:
- If a primary is already established by pathology and it isn't breast, and the clinical impression is a metastasis, it isn't necessary to do IHC if the morphology of the lesion in the liver is compatible with the established primary.
Sign out
Adenocarcinoma
Liver Lesion, Core Biopsy: - ADENOCARCINOMA, POORLY DIFFERENTIATED, see comment. Comment: The tumour consists of round cells with round nuclei and inconspicuous nucleoli. It is in poorly formed glands. The tumour stains as follows: POSITIVE: CK7, CA-125. NEGATIVE: CK20, CDX2, TTF-1. The findings are in keeping with a foregut primary (pancreas, stomach, bile ducts, duodenum). Clinical and radiological correlation is suggested.
Block letters
LIVER, PORTION OF SEGMENTS 2 AND 3, RESECTION: - METASTATIC ADENOCARCINOMA. -- RESECTION MARGIN CLEARANCE 2 MM. - LIVER STEATOSIS, MILD.
Micro
The section show liver parenchyma with an invasive adenocarcinoma. The adenocarcinoma has well formed glands with dirty necrosis. The nuclei are appear crowded and have an ellipsoid shape. Focally, zones of necrosis are present. See background liver.
BACKGROUND LIVER (BASED ON H&E ONLY)
Fibrosis: not identified.
Fibrous septa: absent.
Septa with curved contours: absent.
Large droplet steatosis (% of hepatocytes): mild (20%).
Ballooning of hepatocytes: not identified.
Mallory-Denk bodies: not identified.
Portal inflammation: present, mild.
Interface activity: not identified.
Lobular necroinflammation: not identified.
Ducts: present in normal numbers.
Duct injury: not identified.
Ductular reaction: not identified.
Cholestasis: present peritumoural, otherwise absent.
Terminal hepatic venules: present.
Ground glass cells with routine stains: not identified.
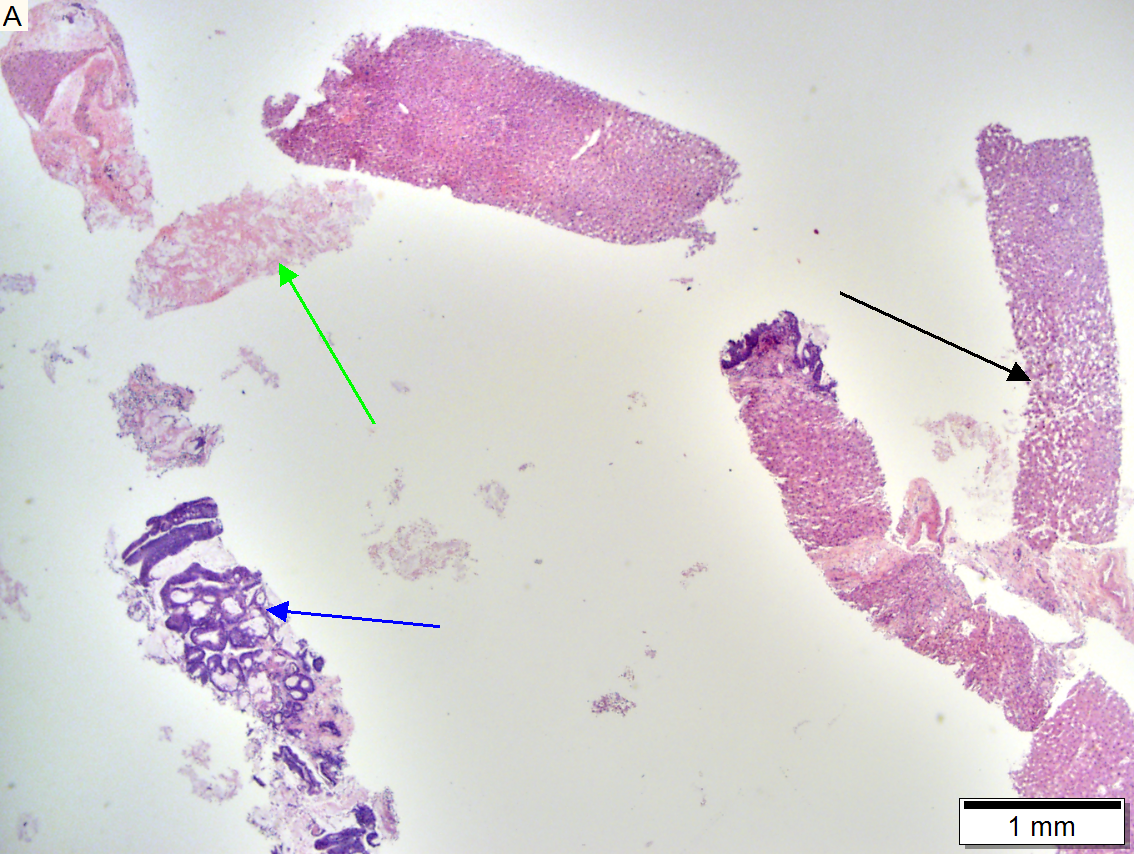
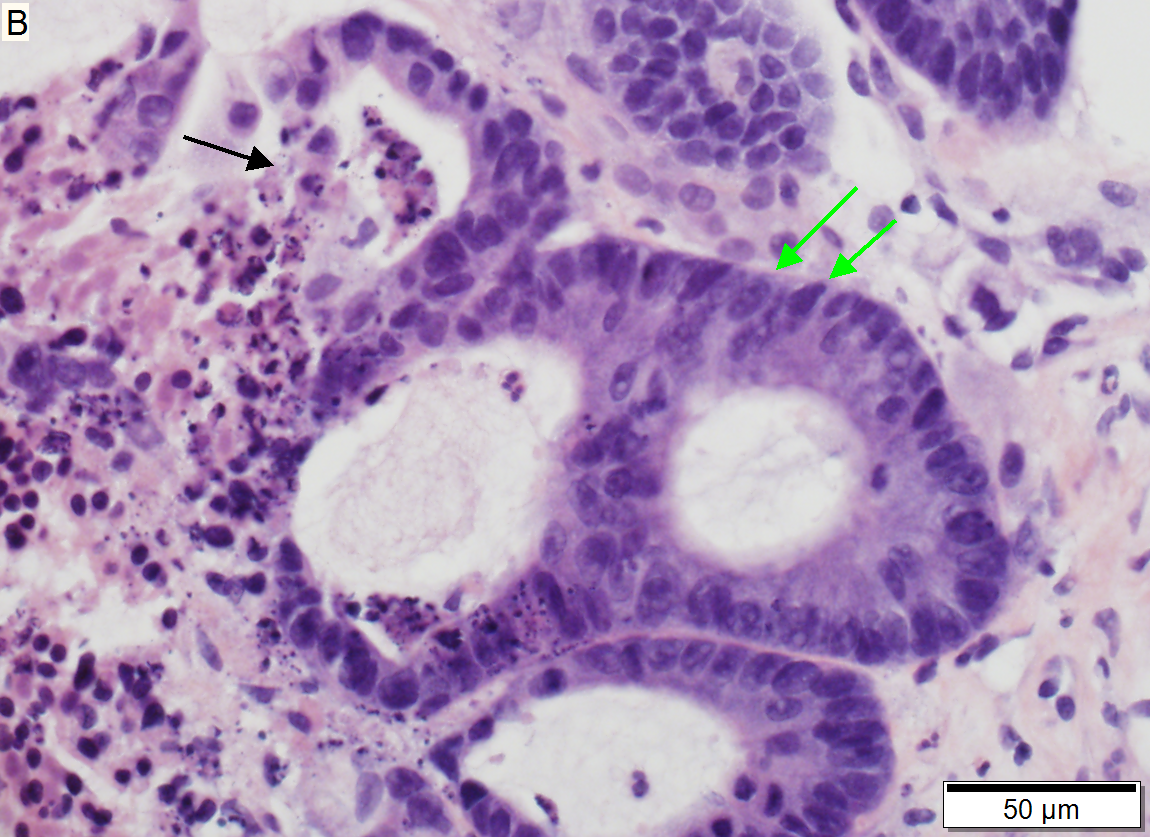
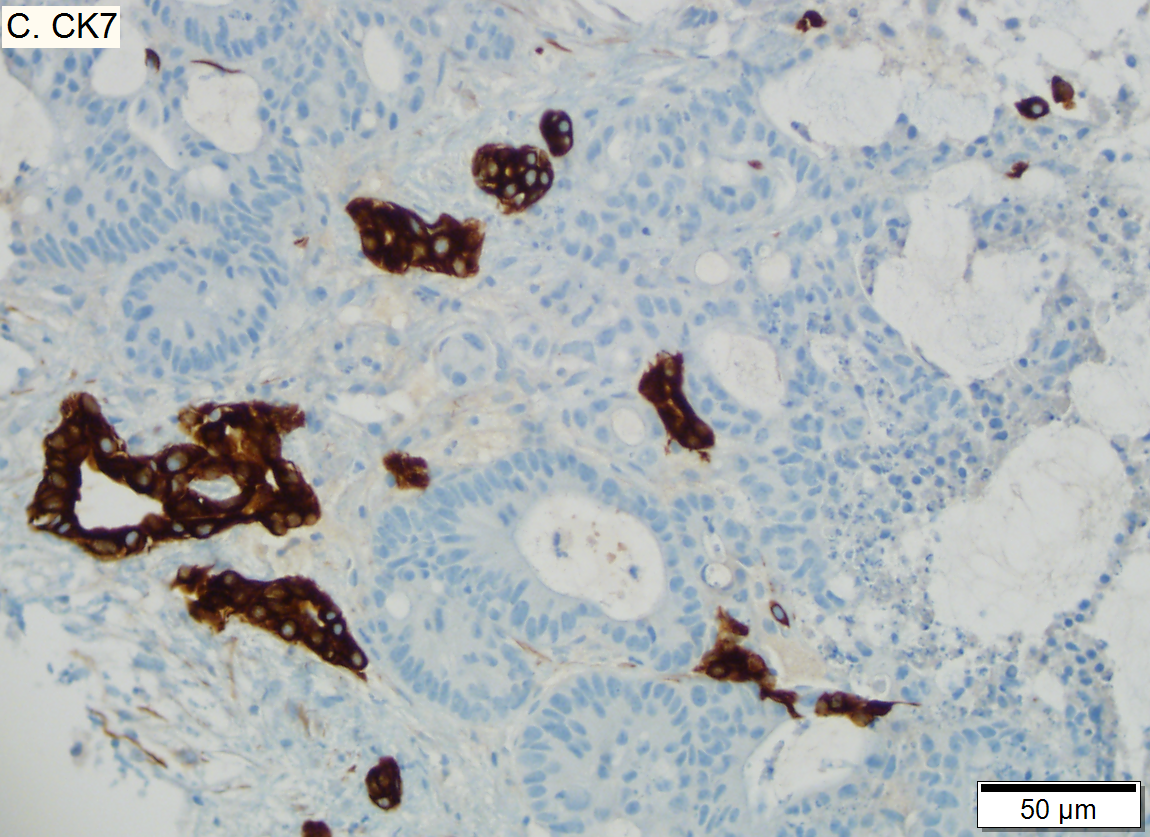
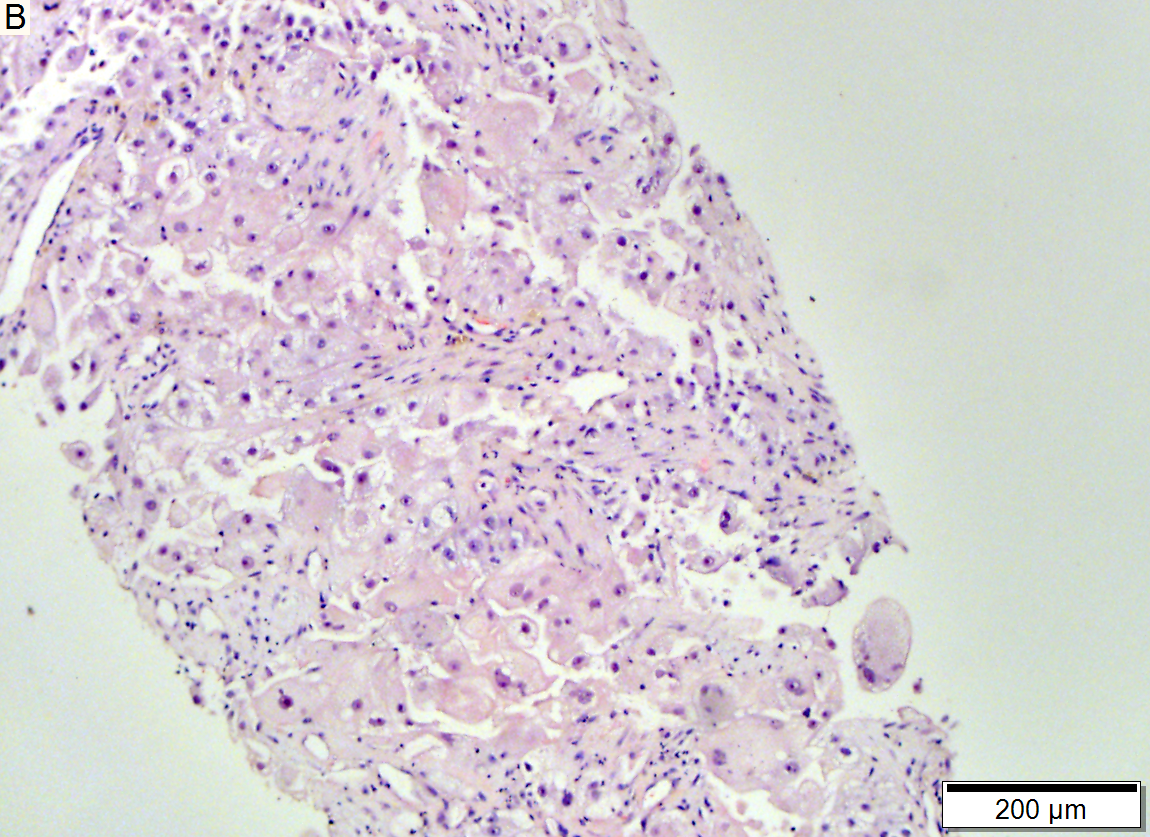
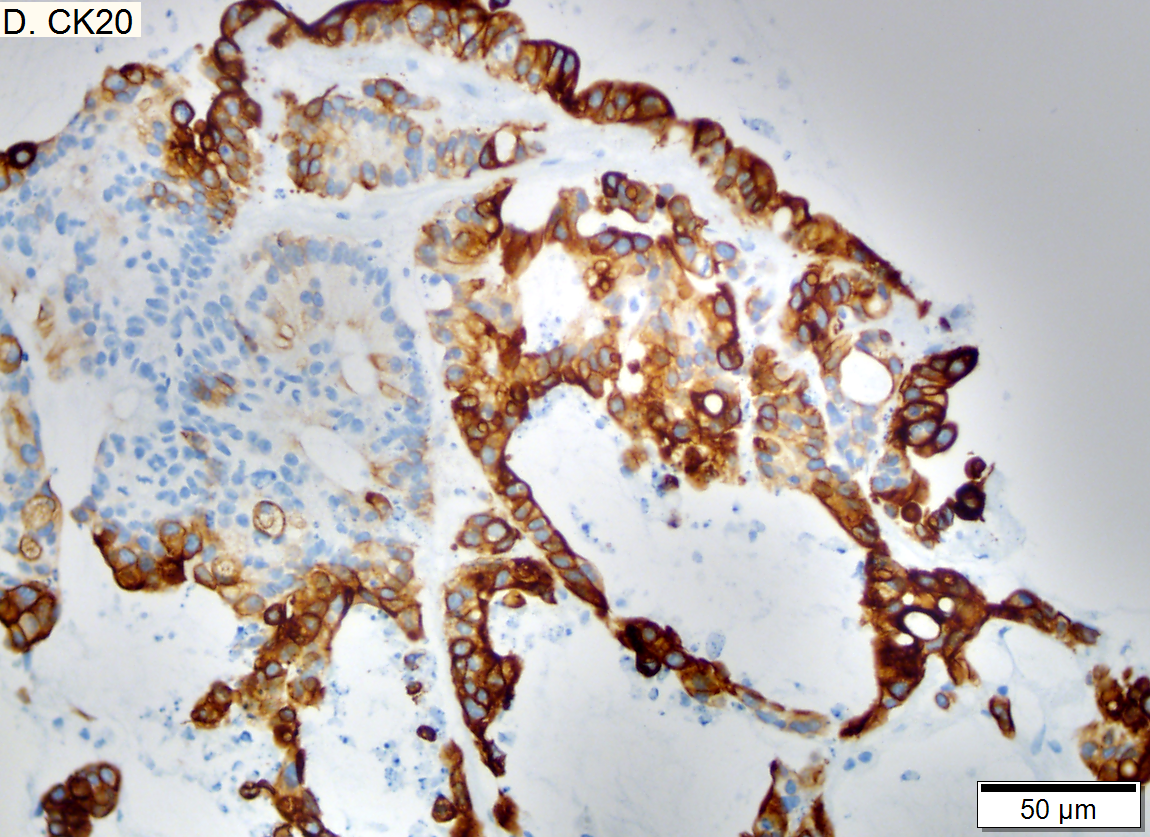 Metastasis of colonic carcinoma to liver
Metastasis of colonic carcinoma to liver
Metastasis of colonic carcinoma to liver. A. Blue arrow shows colon cancer with large acini. Green arrow shows necrotic tumor. Black arrow shows sinusoidal dilatation, an example of a nonspecific tumor associated change that should not be deemed systemic in nature. B. Elongated nuclei perpendicular to acinar border (green arrows) and dirty luminal necrosis (black arrow) are characteristic of colon cancer. C. Immunostain for CK7. Positive groups may be focal staining by tumor, but more likely are residual bile ductules. D. Immunostain for CK20 is more diffusely positive and definitely in tumor.



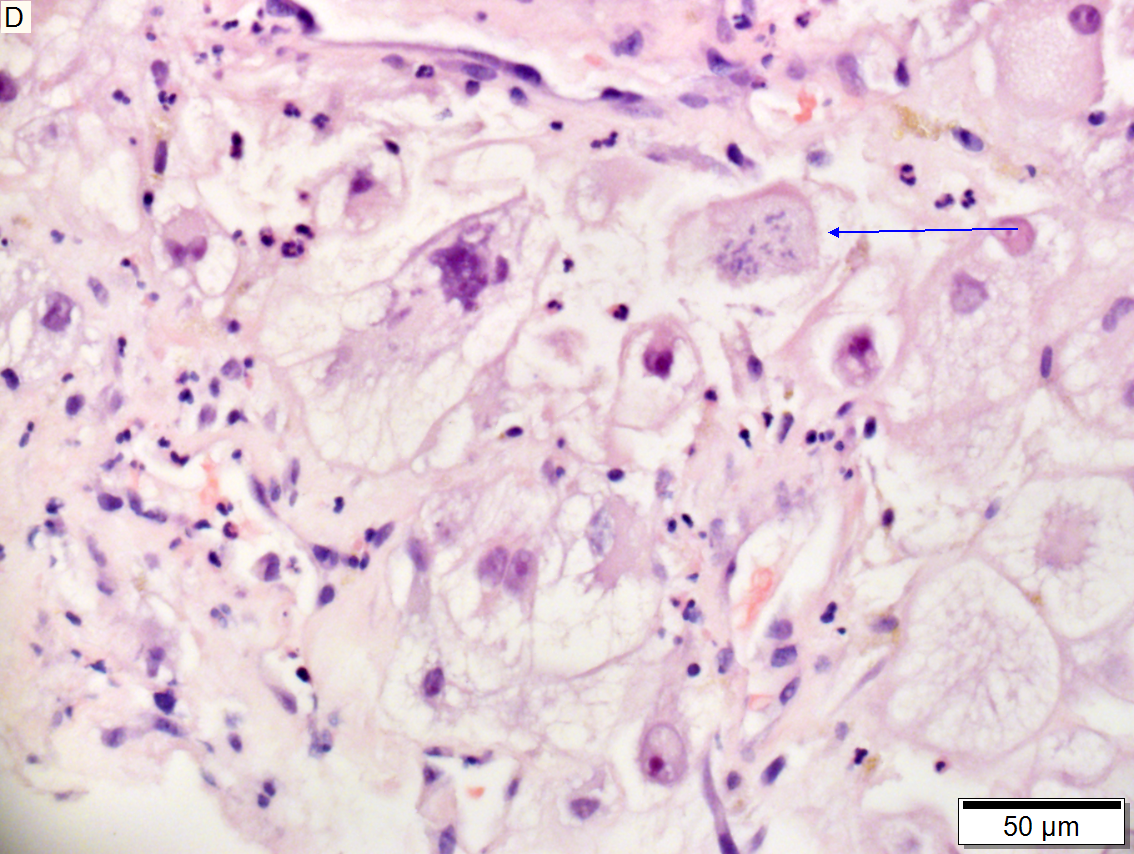
 Metastasis of squamous carcinoma to liver
Metastasis of squamous carcinoma to liver
Metastasis of Squamous carcinoma to liver. A. Dark blue tumor groups mass to replace liver.B. Carcinomatous nests show scant stromal separation, with individual cancer cells being a the edge of the necrotic space C. Huge nucle show nucleoli with occasional spindleds cell nuclei. Individual cell necrosis is prominent. Cytoplasm is scant. Immunostain for CK7. Positive groups may be focal staining by tumor, but more likely are residual bile ductules. D. Immunostain for P63 is positive; CK7, CK20, chromogranin, synaptophysin, and TTF1 were negative
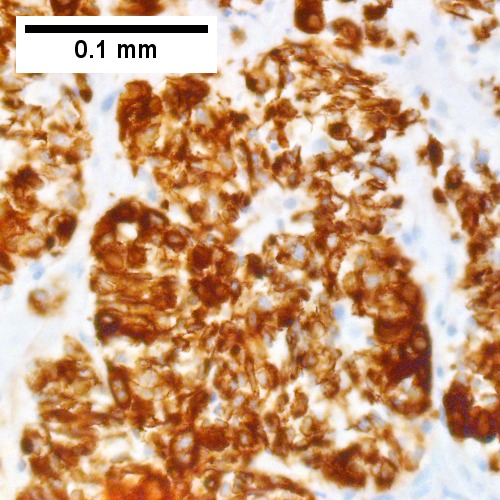
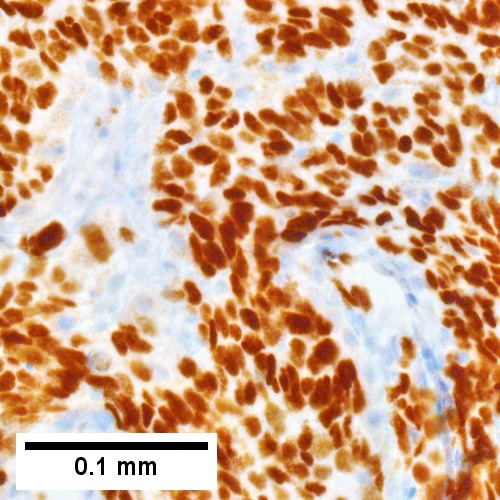
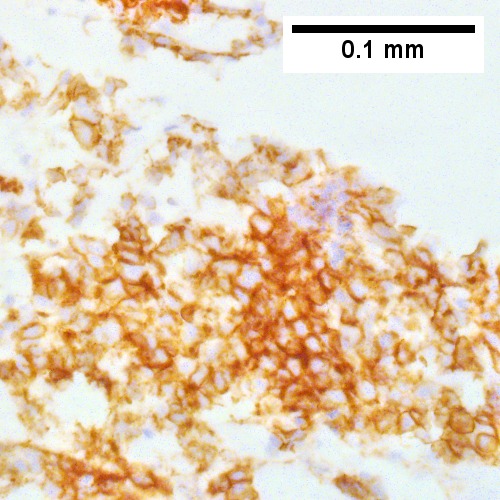
Metastatic poorly differentiated neuroendocrine carcinoma of pulmonary origin. Tumor presents as a mass (Row 1 Left 40X). Tumor replaces most of a triad; note the residual interlobular bile duct [arrow] (Row 1 Right 200X). Tumor cells mold, show scant cytoplasm, and marked anisonucleosis, larger than ordinary small cell carcinoma (Row 2 Left 400X). Tumor cells are positive for CK7 (Row 2 Right 400X). Tumor cells are positive for TTF1 (Row 3 Left 400X). Tumor cells are positive for CD56 (Row 3 Right 400X).
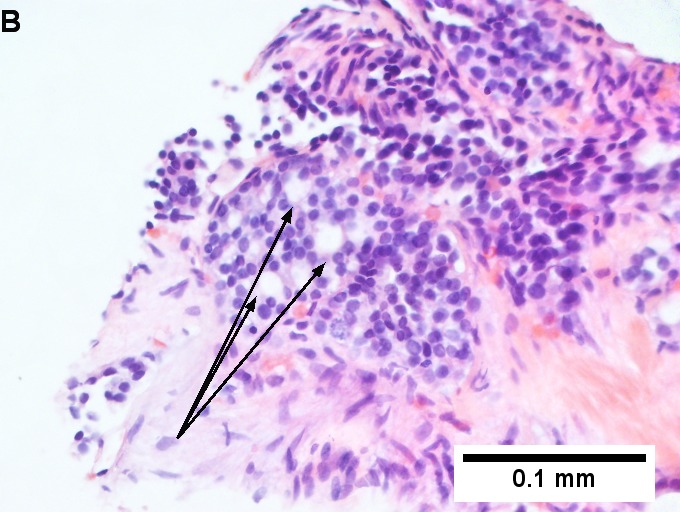
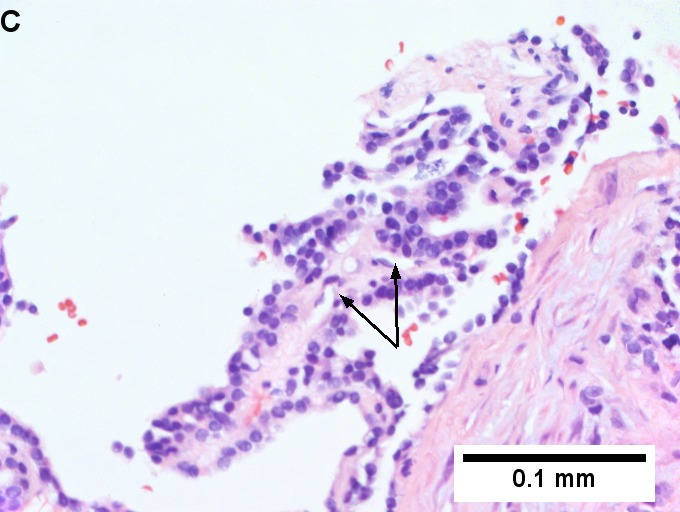
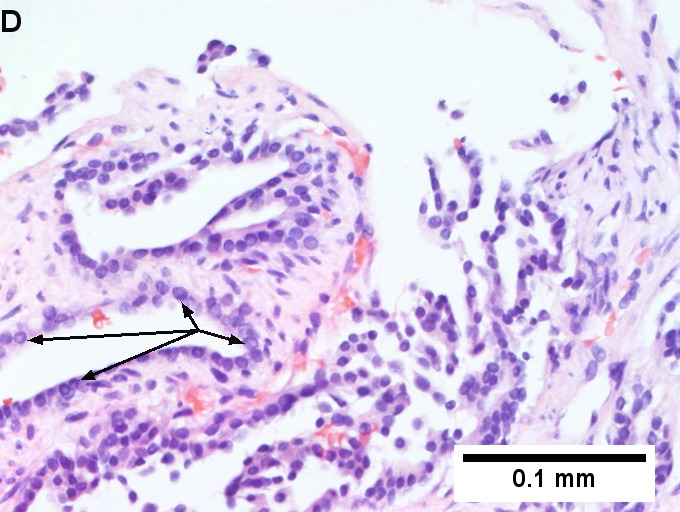
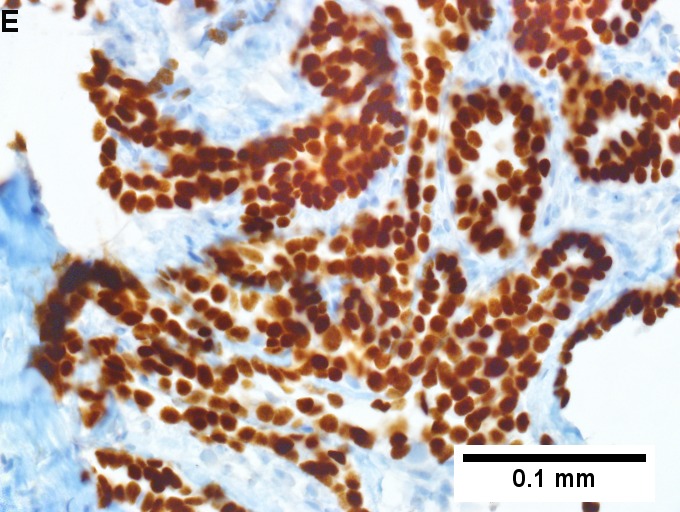
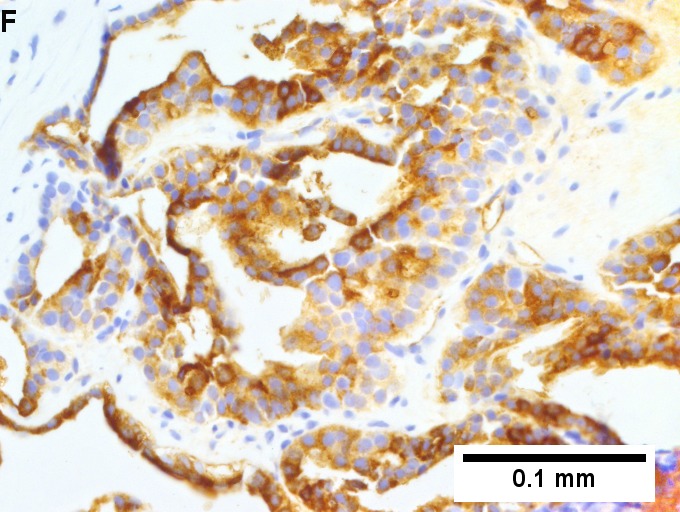
Metastatic thyroid carcinoma. A. Carcinoma with open spaces spares part of a normal liver lobule [arrow] (40X). B. A sheet with acini [arrows] would indicate only adenocarcinoma (400X). C. A true papilla with vascular spaces [arrows] suggests papillary carcinoma (400X). D. Some spaces show tumor nuclei with inclusions [arrows] (400X). E. Tumor nuclei are TTF1 positive, which can also be seen with lung cancer (IHC 400X). F. Specific thyroglobulin positivity rendered the specific diagnosis known (IHC 400X).
Metastasis of renal cell carcinoma to liver. A. Upper core shows blue necrosis; remainder shows replacement by tumor. B. In part cytoplasm is abundant and amphophilic, suggesting hepatocellular carcinoma. C. In part, tumor shows clear cytoplasm, raising alternative explanations. D. High power of clear area shows finely reticulated cytoplasm with sharp intercellular borders. Nuclei are bizarrely shaped, often with nucleoli. Arrow shows an aberrant mitosis. E. Immunostain for RCC proves renal cell carcinoma.
Melanoma
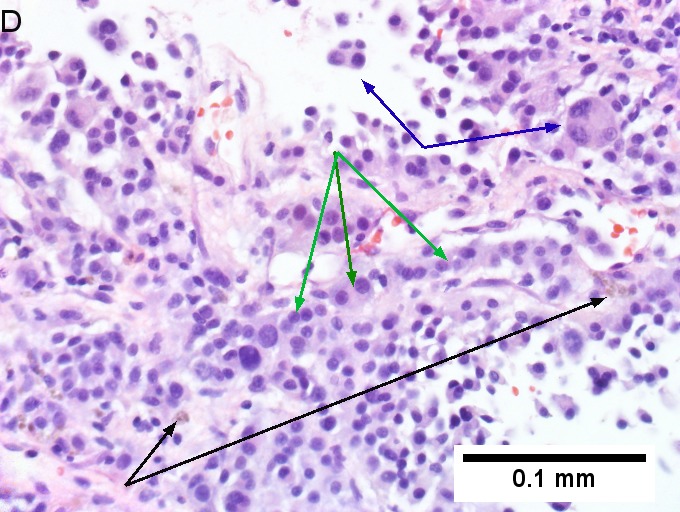
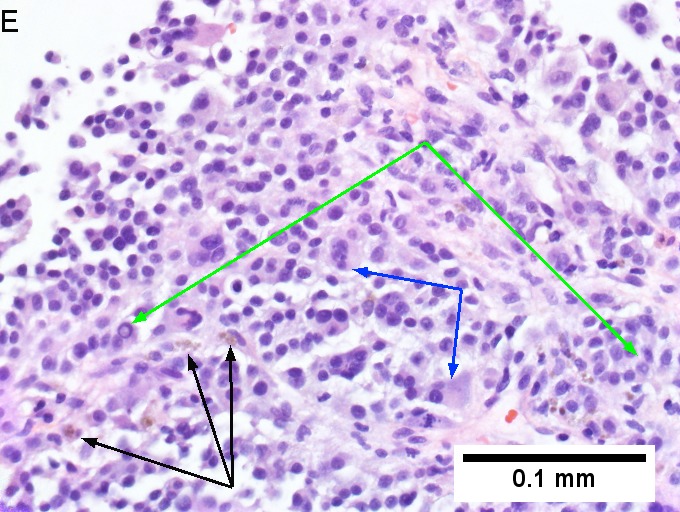
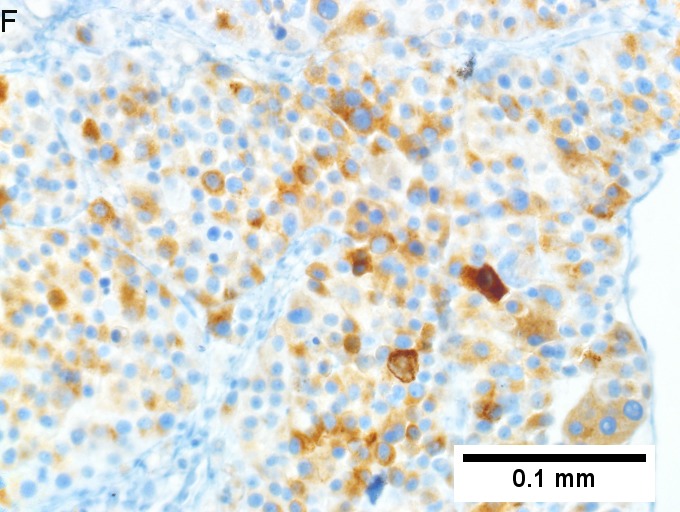
Melanoma metastatic to liver. A. Low power contrasts normal preserved liver (arrow) with remaining mass of cancer [2x]. B. Necrosis on left abuts brown, pigmented cells on right [200X]. C. Apparent acini (green arrows), vacuoles (blue arrow), and papillae (black arrows) might lead to a wrong diagnosis [200X]. D. Apparent wall-like epithelial seeming lining (green arrows) and giant cells (blue arrows) might tempt a wrong diagnosis, which the pigment (black arrows) should discourage [400X]. E. Intranuclear inclusions (green arrow), eccentrically placed nuclei (blue arrows) and pigment (black arrows) suggest the proper diagnosis [400X]. F. MART 1 immunostain is positive in viable cancer cells [400X].
Liver Lesion, Core Biopsy: - METASTATIC MALIGNANT MELANOMA, see comment. Comment: The tumour stains as follows: POSITIVE: S-100, HMB-45, Melan A. NEGATIVE: CK7, CK20, TTF-1. The tumour consists of round cells with round-to-ellipsoid nuclei, inconspicuous nucleoli and a moderate quantity of grey cytoplasm. Nuclear pseudoinclusions are present.
See also
References
- ↑ OA. 29 November 2009.
- ↑ Elias, D.; Rougier, P.; Mankarios, H.; Fahrat, F.; Lasser, P. (Mar 1993). "[Resectable liver metastases and synchronous extra-hepatic sites of colorectal origin. Surgical indications].". Presse Med 22 (11): 515-20. PMID 8511077.
- ↑ Yoon, KH.; Yun, KJ.; Lee, JM.; Kim, CG.. "Solitary necrotic nodules of the liver mimicking hepatic metastasis: report of two cases.". Korean J Radiol 1 (3): 165-8. doi:10.3348/kjr.2000.1.3.165. PMID 11752949.
- ↑ URL: http://www.radiologyassistant.nl/en/p446f010d8f420/liver-masses-i-characterization.html. Accessed on: 4 August 2016.
- ↑ Kanematsu, M.; Kondo, H.; Goshima, S.; Kato, H.; Tsuge, U.; Hirose, Y.; Kim, MJ.; Moriyama, N. (May 2006). "Imaging liver metastases: review and update.". Eur J Radiol 58 (2): 217-28. doi:10.1016/j.ejrad.2005.11.041. PMID 16406434.
- ↑ Kondo, Y.; Nakajima, T. (Sep 1987). "Pseudoglandular hepatocellular carcinoma. A morphogenetic study.". Cancer 60 (5): 1032-7. PMID 2440554.
- ↑ The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.
- ↑ Dennis, JL.; Hvidsten, TR.; Wit, EC.; Komorowski, J.; Bell, AK.; Downie, I.; Mooney, J.; Verbeke, C. et al. (May 2005). "Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm.". Clin Cancer Res 11 (10): 3766-72. doi:10.1158/1078-0432.CCR-04-2236. PMID 15897574.
Further reading
- Anders, RA.; Kamel, IR. (May 2007). "Biopsy considerations in the diagnosis of hepatic masses.". Clin Gastroenterol Hepatol 5 (5): 541-4. doi:10.1016/j.cgh.2007.02.028. PMID 17478344.