Difference between revisions of "Adrenal gland"
(→Adrenocortical carcinoma: split out) |
(split out) |
||
| Line 168: | Line 168: | ||
==Pheochromocytoma== | ==Pheochromocytoma== | ||
{{Main|Pheochromocytoma}} | |||
==Adrenal ganglioneuroma== | ==Adrenal ganglioneuroma== | ||
Revision as of 02:47, 23 August 2014
Adrenal gland is a little organ that hangs-out above the kidney. Pathologists rarely see it. It uncommonly is affected by tumours.
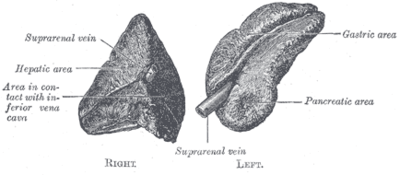
Anatomy & histology
- Adrenal cortical rest redirects here.
Anatomy
- Cortex.
- Medulla.
Microscopic
It is composed of a cortex and a medulla.
Cortex
It has three layers - mnemonic: GFR (from superficial to deep):
- Zona glomerulosa - salt (e.g. aldosterone).
- Eosinophilic cytoplasm. (???)
- Layer normally discontinuous.
- Zona fasciculata - sugar (e.g. cortisol).
- Clear cytoplasm - key feature.
- Largest part of the cortex ~ 70%.
- Cells in cords/nests. (???)
- Zona reticularis - steroid (e.g. dehydroepiandrosterone).
- Marked eosinophilia of cytoplasm - key feature.
- Granular/reticular cytoplasm.
Note:
- Normal cortex may not be completely encapsulated, i.e. the adrenal capsule may have defects.[1]
- In other words: the cortex may "spill" into the surrounding fat.
Medulla
It consists of two cell types:[2]
- Chromaffin cells.
- Arise of neural crest.
- Sustentacular cells (supporting cells).
Produce NED: norepinephrine, epinephrine, dopamine.
Images
www:
IHC
Adrenal cortex:[3]
- Chromogranin A -ve.
- Synaptophysin +ve.
- Alpha-inhibin +ve.
- Vimentin +ve.
- Melan A +ve.
- AE1/AE3 -ve.
Clinical
Patients getting a bilateral adrenalectomy get pre-treatment with steroids.[4]
Adrenal insufficiency is an immediate danger post-op.[5]
Benign
The section covers non-neoplastic pathologies of the adrenal gland. These uncommonly come to the pathologist.
Stress response
- In fetuses - fat content increases due to stress[6] -- see: Fetal_autopsy#Adrenal_fetal_fat_pattern.
- In newborns/children/adults - fat content decreases due to stress.
Spironolactone bodies
Hemorrhagic adrenalitis
- AKA Waterhouse-Friderichsen syndrome.
General
- Classically thought to be only due to Neisseria meningitidis; however, more recently also associated with Staphylococcus aureus,[7] and Streptococcus pneumoniae.[8]
Gross
Features:
- Massive haemorrhage within the substance of the adrenal gland.
DDx (autopsy):
- Post-mortem changes.
Microscopic
Features:
- Massive haemorrhage within the substance of the adrenal gland.
Image: Haemorrhage in adrenal (nih.gov).
Adrenal cytomegaly
General
May be associated with:[9]
- Beckwith-Wiedemann syndrome.
- Prematurity.
- Rh-incompatibility.[10]
Microscopic
Features:
- Large cells in the adrenal cortex.[10]
Addison disease
General
- Chronic adrenocortical insufficiency.
Clinical:
- Brown skin - due POMC (a precursor of ACTH and melanocyte stimulating hormone (MSH)).[11]
- POMC presence implies the pituitary gland intact.
- Hypotension.
- Nausea and vomiting.
DDx:[12]
- Autoimmune.
- Tuberculosis.
- AIDS.
- Malignancy.
Notes:
- Secondary adrenocortical insufficiency (due to pituitary pathology):[13]
- No hyperpigmentation (as no POMC).
- Aldosterone usu. normal.
Microscopic
Features:[11]
- Atrophy adrenal cortex - specifically zona fasciculata and zona reticularis.
Notes:
- There is preservation of zona glomerulosa and medulla.
Benign neoplasms
Adrenal cortical adenoma
General
Epidemiology:
- Often an incidental finding.
Pathologic/clinical:
- May be hormonally active.
- Radiologists are good at identifying adenomas, as they are usually lipid rich and have a characteristic low HU signal.[14]
Indications for excision:[15][16]
- Lesions >30 mm.
- Hormonally active.
- Non-incidental finding. (???)
Notes:
- Cushing disease is due to the ACTH over-production by the pituitary.
- In cortisol producing tumours (Cushing syndrome): atrophy of the non-hyperplastic cortex (due to feedback inhibition from the pituitary gland).
Microscopic
Classic features:
- Well-defined cell borders.
- Clear cytoplasm.
- May have foci of necrosis/degeneration and nuclear atypia.
Note:
- In aldosterone producing tumours:
- May extend outside of the capsule (should not be diagnosed as adrenal cortical carcinoma).
- No atrophy of non-hyperplastic cortex.
DDx:
- Adrenal cortical nodule.[17]
- Adrenal cortical hyperplasia.
- Hyperplasia is multifocal.[18]
- Adrenal cortical carcinoma.
Pheochromocytoma
Adrenal ganglioneuroma
General
- May be retroperitoneal.
- Multiple ganglioneuromas may be due to multiple endocrine neoplasia IIb.
Gross
- Solid.
- White.
- Firm.
- Well-circumscribed.
- May be nodular.
DDx (gross):
Images:
Microscopic
Features:
- Ganglion cells - key feature.
- Large cells with large nucleus.
- Prominent nucleolus.
- Large cells with large nucleus.
- Disordered fibrinous material.
Images:
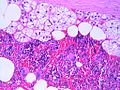
Adrenal myelolipoma
- Myelolipoma redirects here.
General
- Benign and rare.
- Typically asymptomatic and hormonally inactive.[19]
- Symptoms: back or abdominal pain.
- Diagnosis - usu. by abdominal CT.
Treatment:
- Watchful waiting if small (<=7 cm) and asymptomatic.[19]
Microscopic
Features:[20]
- Adipose tissue.
- Hematopoietic elements from all three lineages:
- Erythroid.
- Myeloid.
- Megakaryocytic.
- +/-Calcification.[19]
DDx:[21]
- Angiomyolipoma of the kidney.
- Lipoma.
- Liposarcoma.
- Teratoma.
Images
www:
Adenomatoid tumour
See: Adenomatoid tumours (uterine tumours).
Malignant neoplasms
Adrenocortical carcinoma
- AKA adrenal cortical carcinoma.
- Abbreviated ACC.
Neuroblastoma
- See also: olfactory neuroblastoma.
General
Epidemiology:
- Usually paediatric population.
Laboratory findings:
- Increased urine homovanillic acid.
Predictors of a poor prognosis:[22]
- High mitotic-karyorrhectic index.
- Lack of schwannian stroma.
- >18 months.
- Near ploidy.
- N-MYC amplification.
- Lymph node spread.
- Distant spread.
Classification:
- In a grouping known as neuroblastic tumours which includes:[23]
- Ganglioneuroma (benign).
- Ganglioneuroblastoma (intermediate).
- Neuroblastoma (aggressive).
Gross
- Typically an abdominal mass.
- ~40% arise in the adrenal gland.[24]
Microscopic
Features:[25]
- Small round blue cells separated by thin (pink) fibrous septa.
- Homer-Wright rosettes.
- Rosette with a small (~100 micrometers - diameter) meshwork of fibers (neuropil) at the centre.[26]
- Neuropil-like stroma = paucicellular stroma with a cotton candy-like appearance; see comparison below.
- >50% neuropil-like stroma -- otherwise it's a ganglioneurona or ganglioblastoma.
Notes:
- The fibrous septa are especially useful for differentiation from lymphoma.
DDx:
- Small round cell tumours.
- Wilms tumour.
- Lymphoma.
- Hepatoblastoma.
Images:
Schwannian vs. neuropil
| Feature | Schwannian | Neuropil |
| Cellularity | high ~ spacing of cells < 30 µm | low ~ spacing of cells > 100 µm |
| Fibrillary | yes, long fine strands | no |
| Associations | ganglion cells | neuroblasts |
| Cytoplasmic vacuolation | yes | ? |
Classification/grading
Commonly grouped by the Shimada classification, which depends on the presence a number of things including:
- Mitoses/karyorrhectic cells.
- Molecular abnormalities.
IHC
- PGP 9.5 +ve.[28]
- PGP = protein gene product.
- NB-84 +ve.[29]
- More sensitive that synaptophysin.
- Synaptophysin +ve.
- CD99 -ve.
EM
Distinctive EM appearance:[30]
- Dendritic processes with longitudinally oriented microtubules.
- Membrane bound electron-dense granules (contain catecholamines).
- Desmosomes
- Membrane densities.
Pertinent negative:[30]
- No glycogen.
- Seen in EWS.
See also
References
- ↑ Mills, Stacey E. (2012). Histology for Pathologists (4th ed.). Lippincott Williams & Wilkins. pp. 1236. ISBN 978-1451113037.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1159. ISBN 978-1416031215.
- ↑ De Padua, M.; Rajagopal, V. (May 2008). "Myxoid adrenal adenoma with focal pseudoglandular pattern.". Indian J Med Sci 62 (5): 199-203. PMID 18579979.
- ↑ URL: http://www3.interscience.wiley.com/cgi-bin/fulltext/119909358/PDFSTART. Accessed on: 21 August 2010.
- ↑ URL: http://ats.ctsnetjournals.org/cgi/content/full/62/5/1516. Accessed on: 21 August 2010.
- ↑ Becker MJ, Becker AE (September 1976). "Fat distribution in the adrenal cortex as an indication of the mode of intrauterine death". Hum. Pathol. 7 (5): 495–504. PMID 964978.
- ↑ Adem PV, Montgomery CP, Husain AN, et al. (September 2005). "Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children". N. Engl. J. Med. 353 (12): 1245–51. doi:10.1056/NEJMoa044194. PMID 16177250.
- ↑ Hamilton D, Harris MD, Foweraker J, Gresham GA (February 2004). "Waterhouse-Friderichsen syndrome as a result of non-meningococcal infection". J. Clin. Pathol. 57 (2): 208–9. PMC 1770213. PMID 14747454. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1770213/.
- ↑ URL: http://www.humpath.com/?adrenal-cytomegaly. Accessed on: 3 January 2012.
- ↑ 10.0 10.1 Aterman, K.; Kerenyi, N.; Lee, M. (1972). "Adrenal cytomegaly.". Virchows Arch A Pathol Pathol Anat 355 (2): 105-22. PMID 4336262.
- ↑ 11.0 11.1 Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1157. ISBN 978-1416031215.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1155. ISBN 978-1416031215.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 585. ISBN 978-1416054542.
- ↑ URL: http://emedicine.medscape.com/article/376240-overview.
- ↑ Luton, JP.; Martinez, M.; Coste, J.; Bertherat, J. (Jul 2000). "Outcome in patients with adrenal incidentaloma selected for surgery: an analysis of 88 cases investigated in a single clinical center.". Eur J Endocrinol 143 (1): 111-7. PMID 10870039.
- ↑ Liu, XK.; Liu, XJ.; Dong, X.; Kong, CZ. (Jun 2008). "[Clinical research about treatment for adrenal incidentalomas]". Zhonghua Wai Ke Za Zhi 46 (11): 832-4. PMID 19035218.
- ↑ Thompson, Lester D. R. (2006). Endocrine Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 200. ISBN 978-0443066856.
- ↑ IAV. 18 February 2009.
- ↑ 19.0 19.1 19.2 Daneshmand, S.; Quek, ML. (2006). "Adrenal myelolipoma: diagnosis and management.". Urol J 3 (2): 71-4. PMID 17590837.
- ↑ 20.0 20.1 Cha, JS.; Shin, YS.; Kim, MK.; Kim, HJ. (Aug 2011). "Myelolipomas of both adrenal glands.". Korean J Urol 52 (8): 582-5. doi:10.4111/kju.2011.52.8.582. PMC 3162227. PMID 21927708. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3162227/.
- ↑ Lam, KY.; Lo, CY. (Sep 2001). "Adrenal lipomatous tumours: a 30 year clinicopathological experience at a single institution.". J Clin Pathol 54 (9): 707-12. PMID 11533079.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 254. ISBN 978-1416054542.
- ↑ Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (July 1999). "Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee". Cancer 86 (2): 349–63. PMID 10421272.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 253. ISBN 978-1416054542.
- ↑ Chung EM, Murphey MD, Specht CS, Cube R, Smirniotopoulos JG (2008). "From the Archives of the AFIP. Pediatric orbit tumors and tumorlike lesions: osseous lesions of the orbit". Radiographics 28 (4): 1193–214. doi:10.1148/rg.284085013. PMID 18635637.
- ↑ Wippold FJ, Perry A (March 2006). "Neuropathology for the neuroradiologist: rosettes and pseudorosettes". AJNR Am J Neuroradiol 27 (3): 488–92. PMID 16551982.
- ↑ URL: http://radiographics.rsna.org/content/28/4/1193.full. Accessed on: 12 January 2011.
- ↑ Ootsuka, S.; Asami, S.; Sasaki, T.; Yoshida, Y.; Nemoto, N.; Shichino, H.; Chin, M.; Mugishima, H. et al. (Jun 2008). "Useful markers for detecting minimal residual disease in cases of neuroblastoma.". Biol Pharm Bull 31 (6): 1071-4. PMID 18520032.
- ↑ Miettinen, M.; Chatten, J.; Paetau, A.; Stevenson, A. (Mar 1998). "Monoclonal antibody NB84 in the differential diagnosis of neuroblastoma and other small round cell tumors.". Am J Surg Pathol 22 (3): 327-32. PMID 9500774.
- ↑ 30.0 30.1 Mackay, B.; Masse, SR.; King, OY.; Butler, J. (Dec 1975). "Diagnosis of neuroblastoma by electron microscopy of bone marrow aspirates.". Pediatrics 56 (6): 1045-9. PMID 1196755.